Preparation method and application of porous kieselguhr adsorbing material
An adsorption material, diatomite technology, applied in the field of materials, achieves the effects of low energy consumption, simple and easy preparation method, and large adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of Porous Diatomaceous Earth
[0026] Add acid-treated diatomite and proline into the reactor, start stirring at 200rpm, add thionyl chloride dropwise at -10°C, the mass ratio of porous diatomite and proline varies in the range of 1: 1. The mass ratio of porous diatomaceous earth to thionyl chloride varies from 1:5.8. After the dropwise addition of thionyl chloride, the reaction was continued for 2 hours, then naturally rose to room temperature for 12 hours, and then rotary evaporated at 60° C. for 60 minutes to obtain a porous diatomite adsorption material (I ).
[0027] 2. The application and effect of porous diatomite
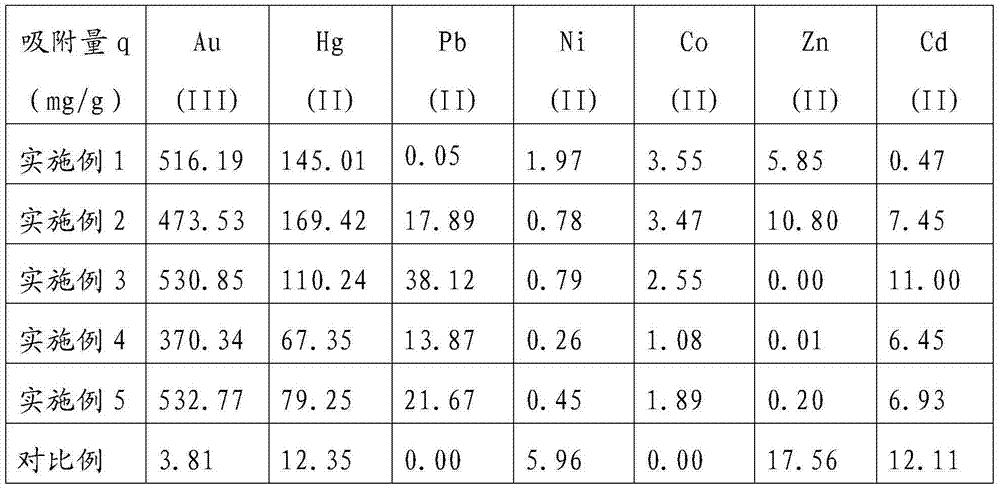
[0028] Weigh the mass of 0.02g of the above-mentioned adsorption material I into a stoppered Erlenmeyer flask, add 2.0×10 -3 mol / L Au(III), Hg(II), Pb(II), Ni(II), Co(II), Zn(II) and Cd(II) aqueous solution 20mL, shake in an air bath shaker for 24 Hour. The concentration of remaining metal ions in the solution was determined by ato...
Embodiment 2
[0031] 1. Preparation of Porous Diatomaceous Earth
[0032] Add acid-treated diatomite and proline into the reactor, start the machine and stir at 220rpm, add thionyl chloride dropwise under the condition of -10°C, the mass ratio of porous diatomite and proline varies in the range of 1: 2. The mass ratio of porous diatomite to thionyl chloride varies from 1:5.8. After the dropwise addition of thionyl chloride was completed, the reaction was continued for 2 hours, then naturally rose to room temperature for 12 hours, and then rotary evaporated at 60°C for 60 minutes to obtain a porous diatomite adsorption material (II ).
[0033] 2. The application and effect of porous diatomite
[0034] Weigh 0.02g of the above-mentioned adsorption material II into a stoppered Erlenmeyer flask, add 2.0×10 -3 mol / L Au(III), Hg(II), Pb(II), Ni(II), Co(II), Zn(II) and Cd(II) aqueous solution 20mL, shake in an air bath shaker for 24 Hour. The concentration of remaining metal ions in the solut...
Embodiment 3
[0037] 1. Preparation of Porous Diatomaceous Earth
[0038] Add acid-treated diatomite and proline into the reactor, start the machine and stir at 250rpm, add thionyl chloride dropwise at -5°C, the mass ratio of porous diatomite and proline varies in the range of 1: 3. The mass ratio of porous diatomaceous earth to thionyl chloride varies from 1:7. After the dropwise addition of thionyl chloride was completed, the reaction was continued for 2 hours, then naturally rose to room temperature for 24 hours, and then rotary evaporated at 70°C for 60 minutes to obtain a porous diatomite adsorption material (III ).
[0039] 2. The application and effect of porous diatomite
[0040] Weigh 0.02g of the above-mentioned adsorbent material III into a stoppered Erlenmeyer flask, add 2.0×10 -3 mol / L Au(III), Hg(II), Pb(II), Ni(II), Co(II), Zn(II) and Cd(II) aqueous solution 20mL, shake in an air bath shaker for 24 Hour. The concentration of remaining metal ions in the solution was deter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com