Synthesis method of Favipiravir
A technology of favipiravir and a synthesis method, applied in the field of drug synthesis, can solve problems such as being unfavorable to industrialized production, and achieve the effects of low production cost, short reaction period and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of compound (III)
[0041]
[0042] Add 50.1g II and 2L methanol to the 3L reaction flask. At 0-5°C, slowly add 133g of 98.3% concentrated sulfuric acid dropwise to it, after the drop is complete, raise the temperature to 40°C, and react for about 48 hours until the reaction of raw material II is basically completed.
[0043] Spin to dry methanol, add 200mL methanol and 500g ice-water mixture to it at 0-5°C, add saturated aqueous sodium bicarbonate solution dropwise thereto to adjust pH=6-7. After suction filtration, the filter cake was vacuum-dried at 45°C for 12 hours to obtain 43.2 g of brown solid III, with a yield of 80.1%.
[0044] MS(m / z):233[M+H] + ; 1 H NMR (DMSO-d 6 ) δ: 3.95(s,3H), 8.45(s,1H), 7.75(s,1H).
Embodiment 2
[0045] Embodiment 2 Preparation of compound (Ⅳ)
[0046]
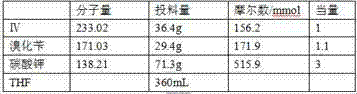
[0047] Add 43g III and 43mL 98.3% concentrated sulfuric acid to a 250mL reaction flask, and stir. At -10~-5°C, add 25.6g of sodium nitrite to it, raise the temperature to 20~25°C after adding, keep stirring for about 0.5h until the reaction of the raw materials is basically completed.
[0048] Slowly add the reaction solution dropwise into 430g of ice water, and keep stirring for about 1.5h until the reaction is complete. After suction filtration, the filter cake was air-dried at 50°C for 6 hours to obtain 36.7 g of orange solid IV with a yield of 85%.
[0049] MS(m / z):232[M-H] - ; 1 H NMR (DMSO-d 6 ) δ: 3.95(s,3H), 8.21(s,1H), 11.52(s,1H).
Embodiment 3
[0050] Embodiment 3 Preparation of compound (Ⅳ)
[0051]
[0052]Add 43g III, 98.3% concentrated sulfuric acid into a 250mL reaction flask, and stir. At -20-0°C, add 12.8g of sodium nitrite to it, raise the temperature to 55-60°C after the addition, keep stirring for about 30 minutes until the raw materials are basically reacted.
[0053] Slowly add the reaction solution dropwise into 430g of ice water, and keep stirring for about 1.5h until the reaction is complete. After suction filtration, the filter cake was air-dried at 50°C for 6 hours to obtain 37.6 g of orange solid IV with a yield of 87%.
[0054] MS(m / z):232[M-H] - ; 1 H NMR (DMSO-d 6 ) δ: 3.95(s,3H), 8.21(s,1H), 11.52(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com