Pharmaceutical composition for treating excessive proliferation diseases
A technology of composition and medicine, applied in the field of pharmaceutical composition containing Ibrutinib and its preparation, and pharmaceutical composition for treating hyperproliferative diseases, which can solve the problems such as no Ibrutinib preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Wet granulation is a widely used method of preparing capsules or compressed tablets. The steps required to prepare capsules or tablets by this method are divided into the following: (1) weighing and mixing the pharmaceutical ingredients; (2) preparing wet granules; (3) sieving the wet granules into pellets or granules, drying the wet granules; (4) Sieve dry granules; (5) Lubricate and mix; (6) Fill into capsules or compress into tablets. In weighing the active ingredient and mixing the active ingredient with the disintegrant, not always the entire amount of the disintegrant is added to the mixture of drug and other excipients, but a portion is reserved, along with the lubricant Add to the prepared drug granules. The granulation is accomplished by adding a granulation solution to the powder mixture, passing the wet material through a sieve of the desired mesh size, drying the granules, and then passing through a second sieve with a smaller mesh size to further reduce th...
specific Embodiment approach
[0046] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0047] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0048] In the present invention, min means minute, mg means milligram, and mL means milliliter.
Embodiment 1
[0050] prescription
[0051] components
Prescription ratio (%)
Prescription amount (mg)
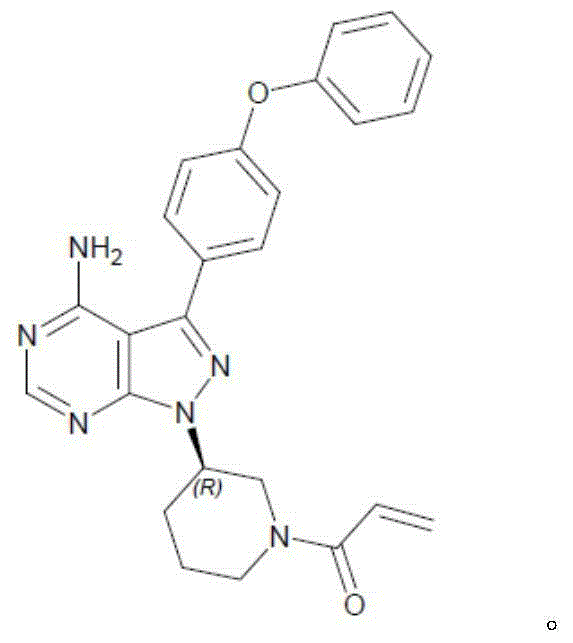
Ibrutinib
75.0
140.0
10.7
20.0
5.8
10.8
6.6
12.3
1.1
2.1
0.8
1.5
[0052] Preparation:
[0053] (1) Mix 140.0mg Ibrutinib, 20.0mg mannitol, 10.8mg microcrystalline cellulose, 12.3mg croscarmellose sodium and 2.1mg sodium lauryl sulfate in a high shear granulator;
[0054] (2) Use pure water as the granulation fluid to granulate;
[0055] (3) drying the granules in a fluidized bed granulator;
[0056] (4) Grinding the dried particles with a vibrating sieve to achieve a suitable particle size;
[0057] (5) Mix the sieved granules with 1.5 mg of magnesium stearate in an appropriate size;
[0058] (6) packed into capsules, and prepared into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com