Synthesis method of D-dibenzoyl tartaric acid
A technology of dibenzoyl tartaric acid and dibenzoyl tartaric anhydride is applied in the field of chemical synthesis of chiral resolution reagents, which can solve the problems of low utilization rate of raw materials, inability to recycle, large production pollution, etc., and achieve convenient operation, The effect of low cost, improved product yield and product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of synthetic method of D-dibenzoyl tartaric acid, it comprises the steps: take D-tartaric acid 150g, toluene 200ml and place 1000ml there-necked bottle, add 1g of copper sulfate under stirring, begin to drop 300g of benzoyl chloride, drip in 2 hours Finished, continue to react for 4 hours, put the material into the centrifuge, and get 438.7g of D-dibenzoyl tartaric anhydride by shaking filter, put it into a 2000ml three-necked bottle, add 438.7g of water and toluene, heat up to reflux, keep for 3 hours, cool down The material was discharged at room temperature, and 344.1 g of D-dibenzoyl tartaric acid was obtained by rejection filtration.
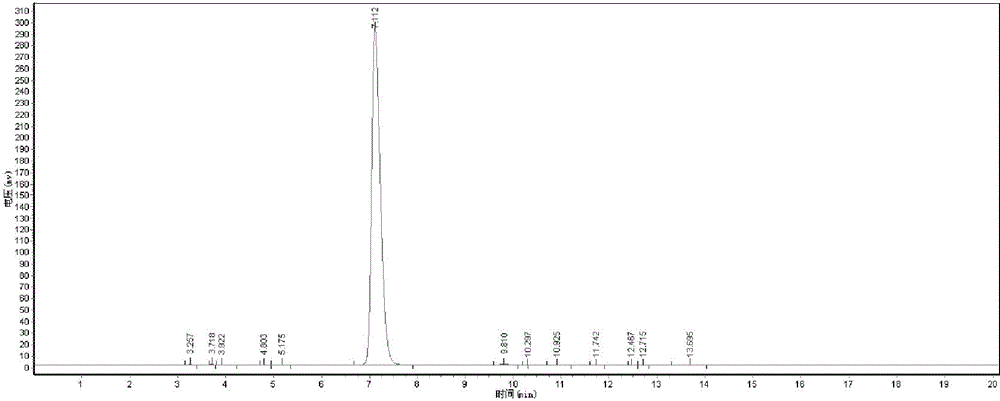
[0036] The D-dibenzoyl tartaric acid that present embodiment obtains is carried out TEM test, test structure such as figure 1 Shown, as can be seen from the figure, the finished product D-dibenzoyl tartaric acid purity content is 99.20%, and the total yield is 95.3%.
[0037] Peak number Peak name Retention time Peak height P...
Embodiment 2
[0052] A kind of synthetic method of D-dibenzoyl tartaric acid, it comprises the steps:
[0053] Put 1.5kg of D-tartaric acid and 2L of toluene into a 10L three-necked bottle, add 11g of copper sulfate under stirring, start to add 3.8kg of benzoyl chloride dropwise, finish dropping in 2.5 hours, continue to react for 4 hours, discharge the material into a centrifuge, and shake off to obtain D-dibenzoyl tartaric anhydride 4.32kg, put it into a 20L three-necked bottle, add water and toluene each 4.32kg, heat up to reflux, keep for 3 hours, cool to normal temperature and discharge, shake off to get D-dibenzoyl tartaric acid 3.45kg kg.
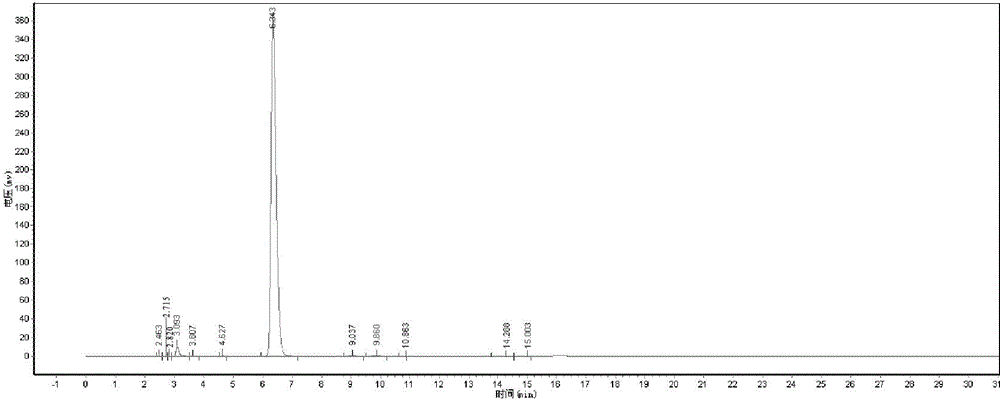
[0054] The D-dibenzoyl tartaric acid that present embodiment obtains is carried out TEM test, test structure such as figure 2 Shown, as can be seen from the figure, the finished product D-dibenzoyl tartaric acid purity content is 99.38%, and the total yield is 95.8%.
[0055] Peak number Peak name Retention time Peak height Peak area Content
...
Embodiment 3
[0069] A kind of synthetic method of D-dibenzoyl tartaric acid, it comprises the steps:
[0070] Put 7.5kg of D-tartaric acid and 10L of toluene into a 50L reactor, add 45g of ferrous sulfate under stirring, start to add 18kg of benzoyl chloride dropwise, finish dripping in 3.5 hours, continue to react for 4 hours, discharge the material to a centrifuge, and shake off to obtain D-dibenzoyl tartaric anhydride 20.7kg, put it into a 100L reactor, add water and toluene each 20.7kg, heat up to reflux, keep for 3 hours, cool down to normal temperature and discharge, shake off to get D-dibenzoyl tartaric acid 17.1 kg.
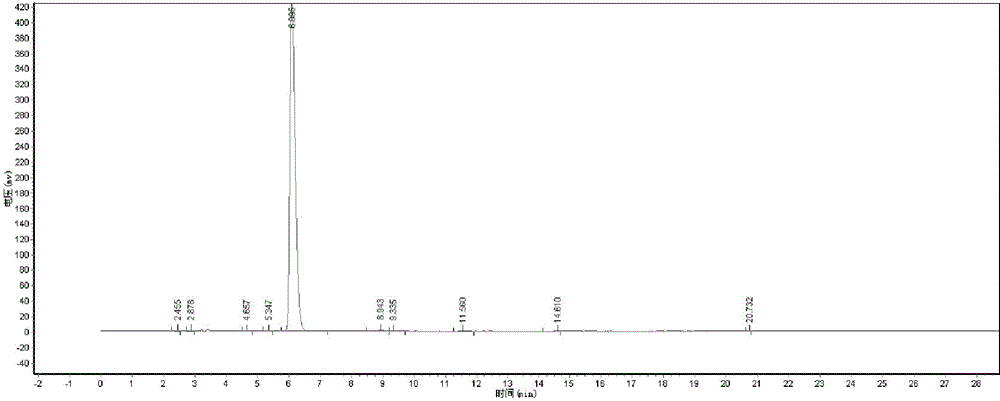
[0071] The D-dibenzoyl tartaric acid that present embodiment obtains is carried out TEM test, test structure such as image 3 Shown, as can be seen from the figure, the finished product D-dibenzoyl tartaric acid purity content is 99.05%, and the total yield is 95.5%.
[0072] Peak number Peak name Retention time Peak height Peak area Content
[0073] 1 2.455 266.13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com