Genetically engineered bacterium capable of expressing cell-penetrating peptide fusion protein and application
A technology for genetically engineering bacteria and proteins, applied in the field of genetic engineering, can solve the problems of asynchrony of virus speed, decrease of virus activity, increase of virus titer, etc., and achieve the effects of convenient large-scale production, low cost and simple operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
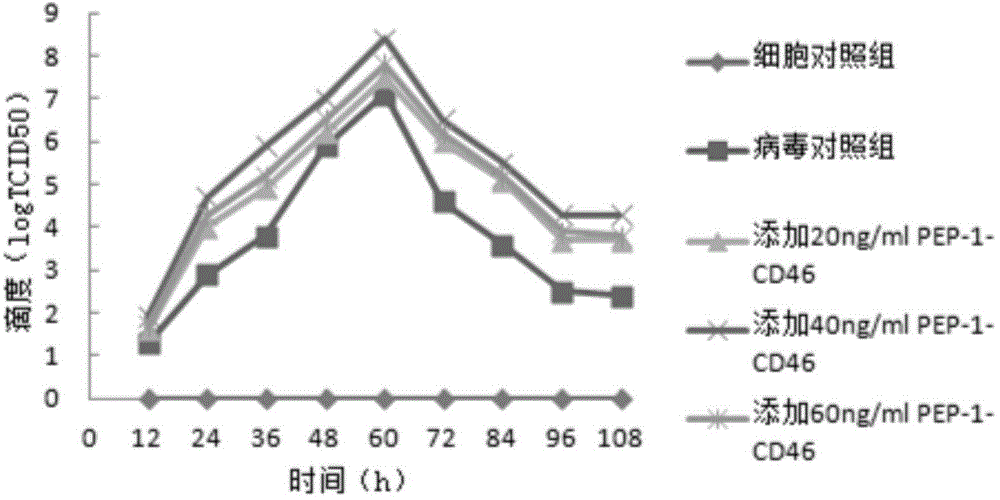
Image
Examples
Embodiment 1
[0032] Embodiment 1: Artificial synthesis of PEP-1-CD46 gene
[0033] According to the sequence of CD46 (Gene ID: 396922) and PEP-1 published in GeneBank, the PEP-1-CD46 gene was artificially synthesized, and EcoR I and BamHI restriction sites were added to its two ends, and the synthetic gene sequence Contains the coding region sequence shown in SEQ ID NO.1.
Embodiment 2
[0035] Construction of recombinant plasmid pET-32a-PEP-1-CD46
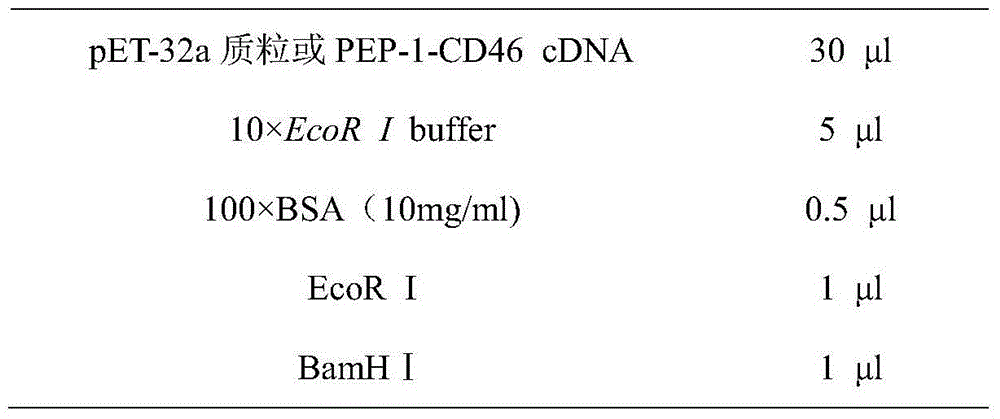
[0036] The vector pET-32a plasmid and PEP-1-CD46 cDNA were digested with restriction endonucleases EcoR I and BamHI. The enzyme digestion system is as follows:
[0037]
[0038]
[0039] The enzyme digestion reaction condition is 37° C., and the reaction time is 2 to 3 hours. The digested products were subjected to agarose gel electrophoresis, and the open-loop pET-32a and PEP-1-CD46 cDNA digested fragments were recovered with a gel recovery kit.
[0040] The PEP-1-CD46 cDNA fragment was ligated with the open-circle pET-32a to obtain the recombinant plasmid pET-32a-PEP-1-CD46. The connection system is as follows:
[0041]
[0042] The ligation reaction solution was mixed well, ligated at 22°C for 2 hours, and stored at 4°C for use.
[0043] Calcium chloride method prepares Escherichia coli competent cell, and its steps are:
[0044] 1) Pick a single colony of DH5α on a fresh solid LB plate, inoculate...
Embodiment 3
[0068] Embodiment 3: the preparation of escherichia coli genetic engineering bacterium
[0069] Plasmid pET-32a-PEP-1-CD46 (1ul) was transformed into Escherichia coli BL21 (DE 3 ) competent cells. Positive transformants were screened out through PCR identification, and the resulting positive clone was the recombinant genetic engineering strain Escherichia coli BL21 (DE 3 ) pET-32a-PEP-1-CD46. The bacterium was deposited on December 5, 2014 and sent to the China Center for Type Culture Collection for preservation, the preservation number is CCTCC NO: M2014631, address: Wuhan University, Wuhan, China, classification name: Escherichia coli BL21(DE3) / pET- 32a – CSFR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com