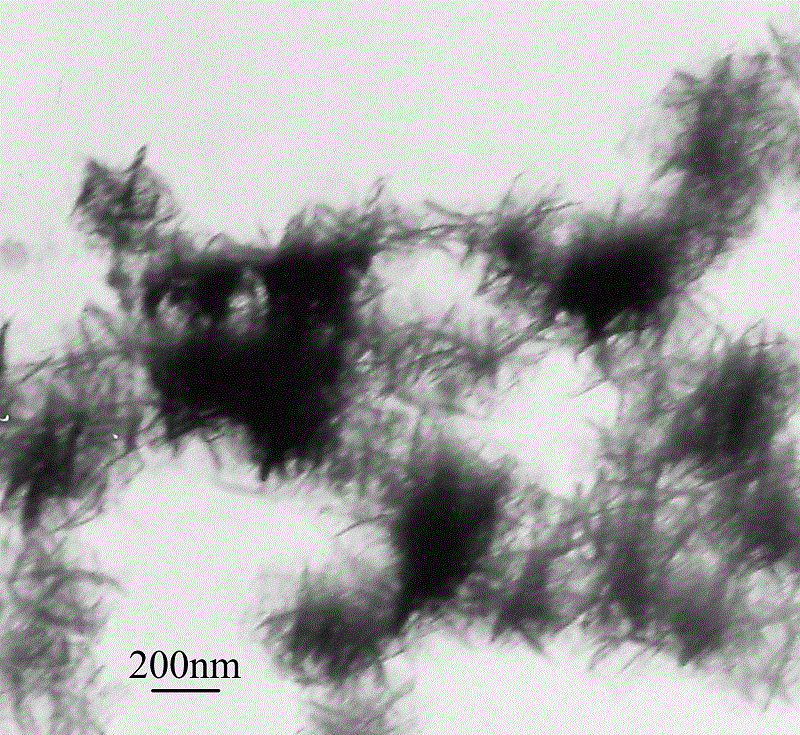
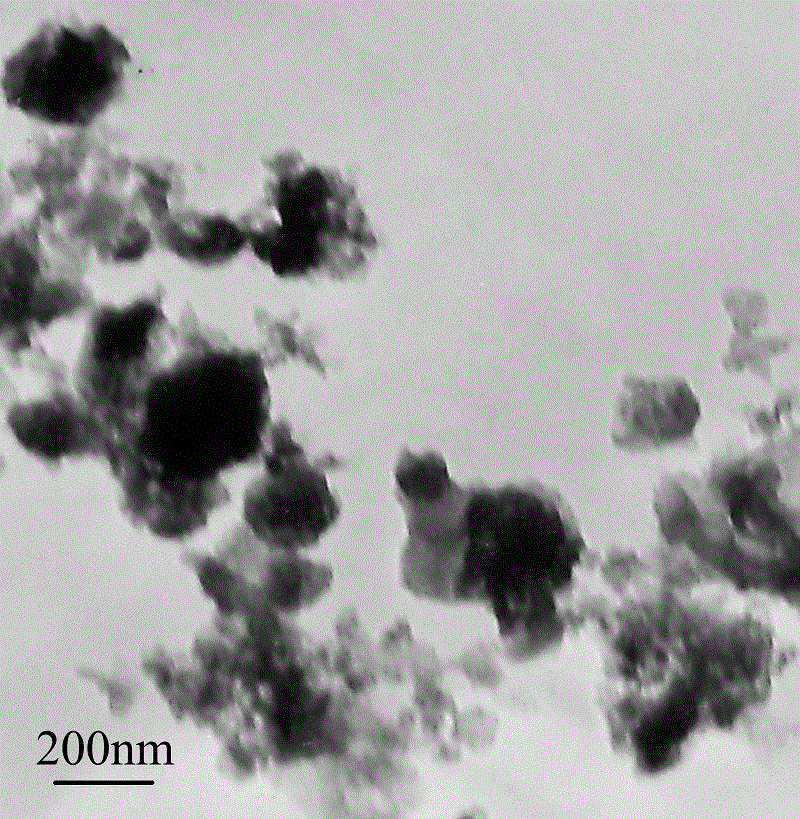
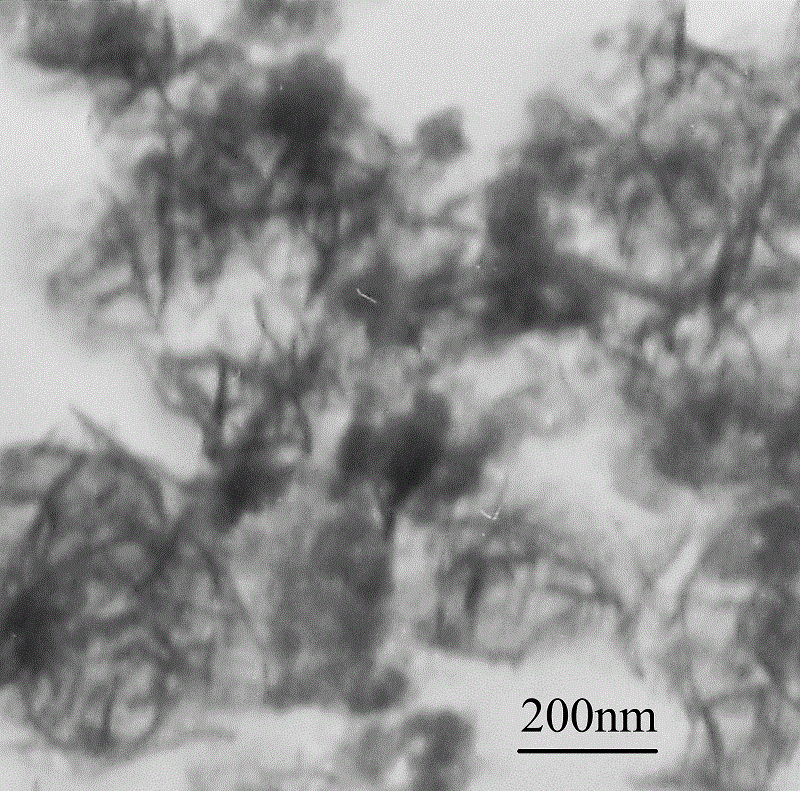
A kind of preparation method of solid barbed spherical conductive copolymer
A technology of spine balls and copolymers, which is applied in the field of preparation of conductive copolymer spine balls, and achieves the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Under the condition of stirring at 300 r / min, dissolve 5.8mmol of ferrous chloride in 150mL deionized water in a three-necked flask to obtain a concentration of 3.8×10 -2 mol / L ferrous chloride solution, stirred evenly and placed in a cold bath for later use.
[0032] (2) Add 0.23mL of pyrrole monomer to the uniformly stirred ferrous chloride solution, and continue stirring for 30min to make the solution evenly mixed.
[0033] (3) Add 0.34mL aniline monomer to the mixed solution of ferrous chloride and pyrrole monomer that is stirred evenly, and continue to stir for 30min to make the solution mix evenly.
[0034] (4) Slowly add cumene hydroperoxide solution dropwise to the mixed solution under stirring condition, and react at about 0ºC for 24 hours to obtain a brown reaction solution.
[0035] (5) The obtained reaction solution was subjected to solid-liquid separation, and the liquid was recovered for use; the solid was washed with water and alcohol three times, an...
Embodiment 2
[0038] (1) Under the condition of stirring at 300 r / min, dissolve 5.8mmol of ferrous chloride in 150mL deionized water in a three-necked flask to obtain a concentration of 3.8×10 -2 mol / L ferrous chloride solution, stirred evenly and placed in a cold bath for later use.
[0039] (2) Add 0.16mL of pyrrole monomer to the uniformly stirred ferrous chloride solution, and continue stirring for 30min to make the solution evenly mixed.
[0040] (3) Add 0.46 mL of aniline monomer to the mixed solution of ferrous chloride and pyrrole monomer that is stirred evenly, and continue stirring for 30 minutes to make the solution mix evenly.
[0041] (4) Slowly add cumene hydroperoxide solution dropwise to the mixed solution under stirring condition, and react at about 0ºC for 24 hours to obtain a brown reaction solution.
[0042] (5) The obtained reaction solution was subjected to solid-liquid separation, and the liquid was recovered for use; the solid was washed with water and alcohol three...
Embodiment 3
[0044] (1) Under the stirring condition of 500 r / min, dissolve 5.8mmol of ferrous chloride in 150mL deionized water in a three-necked flask to obtain a concentration of 3.8×10 -2 mol / L ferrous chloride solution, stirred evenly and placed in a cold bath for later use.
[0045] (2) Add 0.28mL of pyrrole monomer to the uniformly stirred ferrous chloride solution, and continue to stir for 30min to make the solution evenly mixed.
[0046] (3) Add 0.27mL aniline monomer to the mixed solution of ferrous chloride and pyrrole monomer that is stirred evenly, and continue stirring for 30min to make the solution mix evenly.
[0047] (4) Slowly add cumene hydroperoxide solution dropwise to the mixed solution under stirring condition, and react at about 0ºC for 24 hours to obtain a brown reaction solution.
[0048] (5) The obtained reaction solution was subjected to solid-liquid separation, and the liquid was recovered for use; the solid was washed with water and alcohol three times, and d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com