Application of pyrazole hydrazone derivative in preparation of anti-breast cancer drug
An anti-breast cancer, breast cancer technology, applied in drug combinations, anti-tumor drugs, organic chemistry, etc., to achieve the effect of inhibiting growth and good development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (E)-N-(4-methoxybenzylidene)-1-(3-(6-chloropyridine)methyl)-3-(4-chlorophenyl)-1H-pyrazole-5- Preparation of carbohydrazide
[0032]1) Add 0.690 gram of potassium carbonate (0.005 mol), 1.253 gram of 3-(4-chlorophenyl)-1H-pyrazole-5-carboxylic acid ethyl ester (0.005 mol), 0.810 gram of 2 -Chloro-5-chloromethylpyridine (0.005 mol) and acetonitrile (25 ml), equipped with a reflux condenser, the upper part is connected to a drying tube. Heat to reflux for 2 hours, react until the raw material is completely consumed, and detect the end point of the reaction by TLC. Concentrate under reduced pressure, remove the solvent, add ethyl acetate (30 ml), filter, concentrate the filtrate, and use ethyl acetate-petroleum ether (V / V=1 / 2) as the eluent to separate the residue by silica gel column chromatography (100~ 200 mesh silica gel) to obtain ethyl 1-(3-(6-chloropyridine)methyl)-3-(4-chlorophenyl)pyrazole-5-carboxylate with a yield of 90%.
[0033] 2) Add 1.2 mL of 80% Hydraz...
Embodiment 2
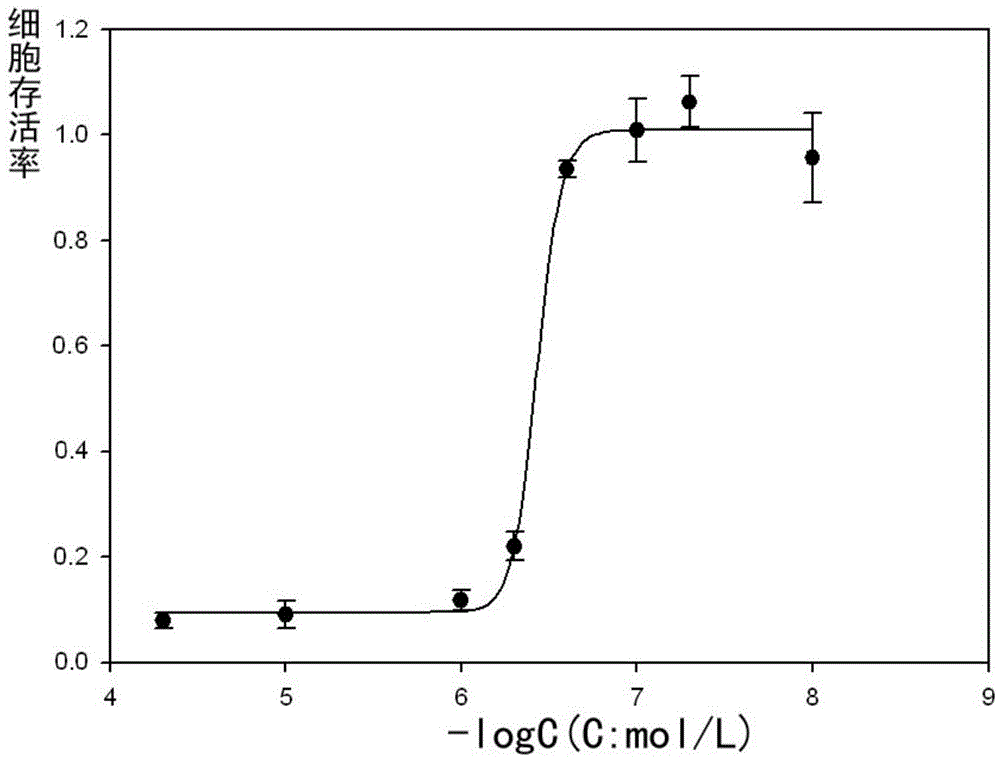
[0048] Determination of (E)-N-(4-methoxybenzylidene)-1-(3-(6-chloropyridine)methyl)-3-(4-chlorophenyl)-1H-pyrazole by SRB method -5-carbohydrazide half inhibitory concentration on growth of breast cancer cells
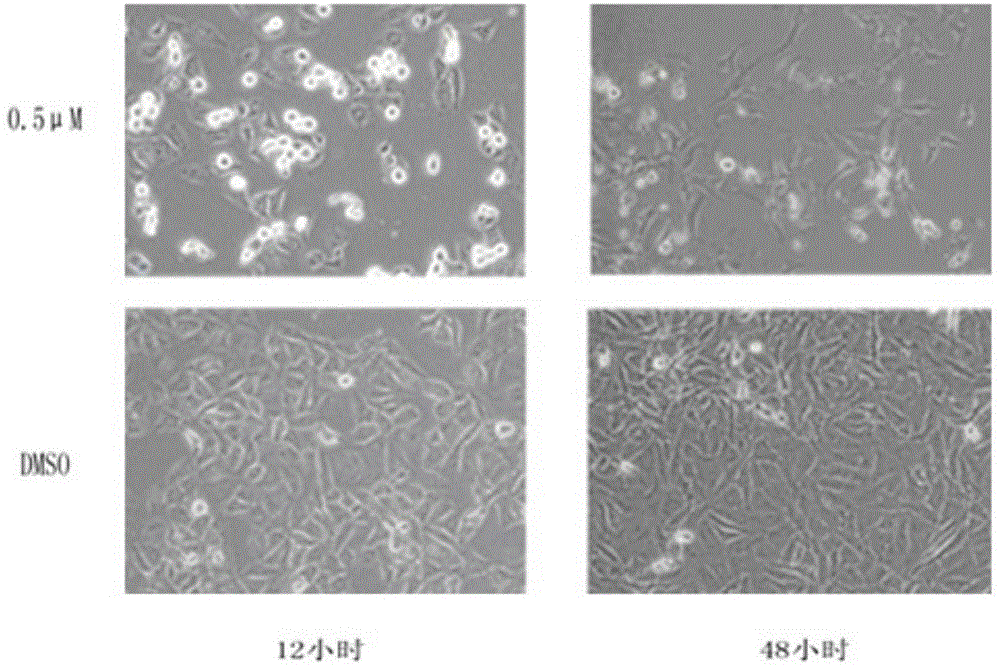
[0049] at 37°C with 5% CO 2 MDA-MB231 breast cancer cells were cultured with DMEM / 10% fetal bovine serum medium under the environment of . MDA-MB-231 breast cancer cells in logarithmic phase were collected, seeded in 96-well plates, and incubated for 24 hours. Add different concentrations (50, 10, 1, 0.5, 0.25, 0.1, 0.05, 0.01 μM) of (E)-N-(4-methoxybenzylidene)-1-(3-(6-chloropyridine ) methyl)-3-(4-chlorophenyl)-1H-pyrazole-5-carbohydrazide was incubated for 48 hours, then 50 μL of trichloroacetic acid solution (30%) was added and fixed at 4° C. for one hour. Shake off the solution, rinse with high-purity water five times, add 100 μL sulforhodamine B to stain for 30 minutes after drying, rinse with 1% acetic acid solution five times after drying. After drying, add...
Embodiment 3
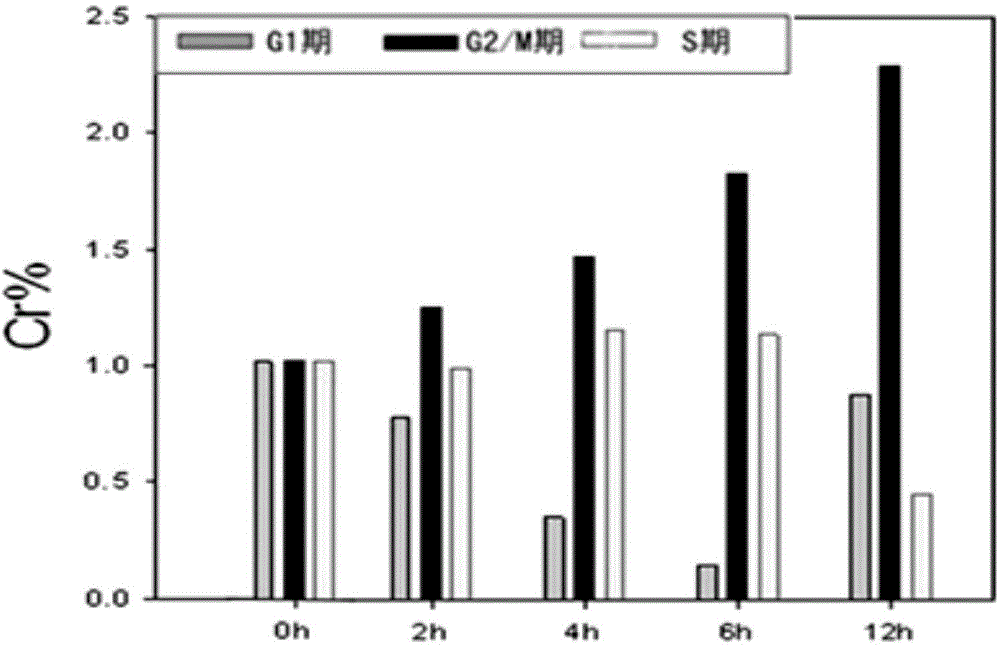
[0052] Determination of (E)-N-(4-methoxybenzylidene)-1-(3-(6-chloropyridyl)methyl)-3-(4-chlorophenyl)- by PI staining flow cytometry Effect of 1H-pyrazole-5-carbohydrazide on the cell cycle of breast cancer
[0053] The MDA-MB-231 breast cancer cells in the logarithmic growth phase were inoculated into 6 cm culture dishes according to the number of 200,000 cells in 3 mL of medium volume, and a total of 16 culture dishes were inoculated. After 24 hours, 16 Petri dishes were equally divided into four groups. Among the four culture dishes in each group, two culture dishes were added with DMSO, and two culture dishes were added with 2.0 μM compound (E)-N-(4-methoxybenzylidene)-1-(3-(6-chloro pyridyl)methyl)-3-(4-chlorophenyl)-1H-pyrazole-5-carbohydrazide. After the four groups were incubated for 2h, 4h, 6h, and 12h, the cells were digested on ice, and the cell suspension was collected, centrifuged, and washed twice with PBS. Add 10 ml of 70% ice ethanol to suspend at -20°C over...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com