Organic electroluminescent material, preparation method of organic electroluminescent material and organic electroluminescent device
A luminescent and electromechanical technology, applied in the field of phosphorescent materials, can solve the problems of blue phosphorescent materials, luminous color purity, luminous efficiency, device efficiency attenuation bottleneck, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
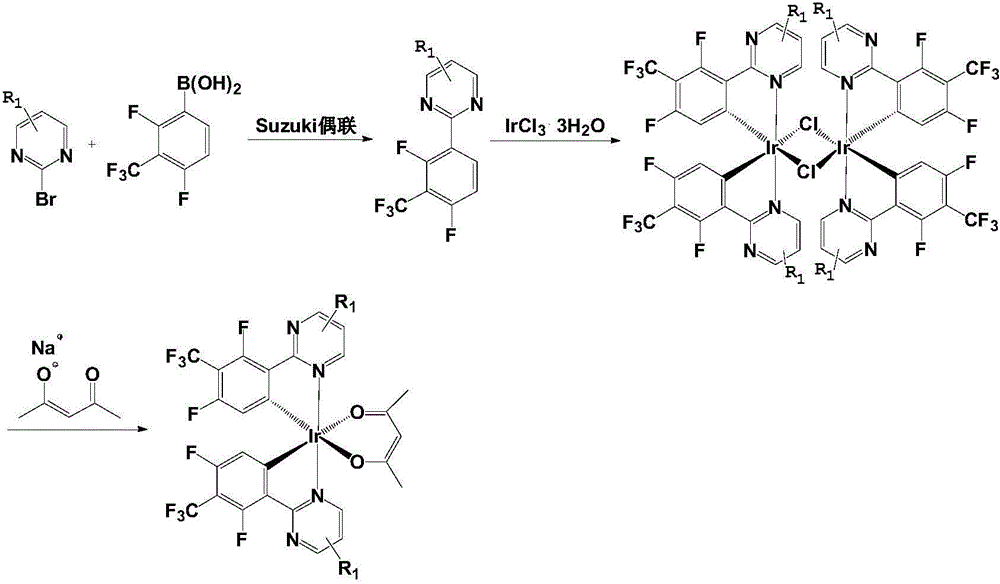
[0048] The preparation process of the organic electroluminescent material (P) of the present invention is roughly divided into the following steps:
[0049] (1) Synthesize compound C through Suzuki coupling reaction of compound E and compound F; wherein, compound F is 2,4-difluoro-3-trifluoromethylphenylboronic acid, and the structural formulas of compound E and compound C are as follows:
[0050] Compound E is Compound C is Among them, R 1 is a hydrogen atom, C 1~20 straight chain alkyl, C 1~20 Branched chain alkyl, C 1~20 Linear alkoxy or C 1~20 branched chain alkoxy.
[0051] (2) Reaction of compound C prepared in step (1) with compound D to generate a chlorine-bridged dimer, namely compound A. Wherein, compound D is trihydrate iridium trichloride IrCl 3 ·3H 2 O. The structural formula of compound A is as follows:
[0052]
[0053] (3) Compound A prepared in step (2) is used as a ring metal ligand, compound B is used as an auxiliary ligand source, and compou...
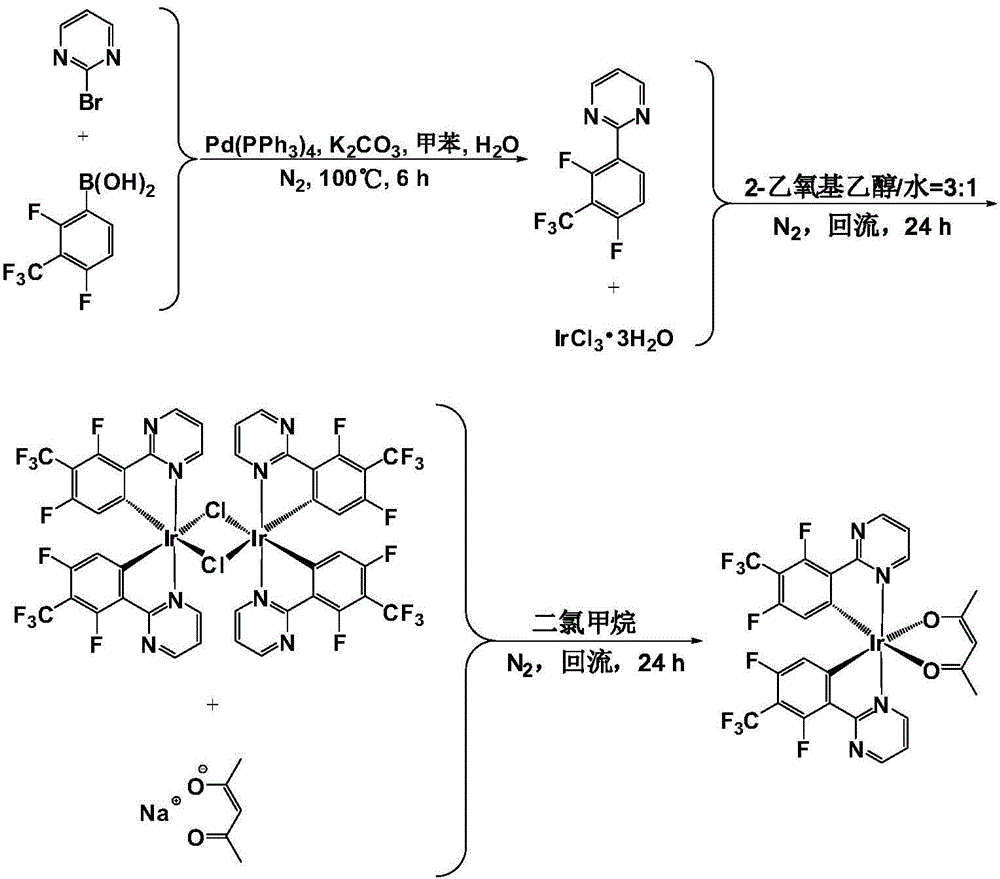
Embodiment 1
[0058] The organic electroluminescent material (P) disclosed in this example is the complex bis(2-(4′,6′-difluoro-5′-trifluoromethylphenyl)pyrimidine-N,C 2 ') (acetylacetonate) iridium, its structural formula is as follows:
[0059]
[0060] It is prepared by the following steps:
[0061] (1) Synthesis of 2-(2′,4′-difluoro-3′-trifluoromethylphenyl)pyrimidine
[0062] The reaction formula is as follows:
[0063]
[0064] The specific steps are: under nitrogen atmosphere, 1.59g (10mmol) 2-bromopyrimidine, 2.71g (12mmol) 2,4-difluoro-3-trifluoromethylphenylboronic acid and 0.58g (0.5mmol) tetrakis (triphenyl Phosphorus) palladium was dissolved in 40ml of toluene, stirred for 10min. Subsequently, 20 ml of an aqueous solution containing 2.76 g (20 mmol) of potassium carbonate was added dropwise to the reaction system. Heat to 100°C and stir the reaction for 6h. After the reaction solution was cooled to room temperature, it was extracted with dichloromethane, separated, w...
Embodiment 2
[0081] The organic electroluminescent material disclosed in this example is the complex bis(2-(4′,6′-difluoro-5′-trifluoromethylphenyl)-5-methylpyrimidine-N,C2 ') (acetylacetonate) iridium, its structural formula is as follows:
[0082]
[0083] It is prepared by the following steps:
[0084] (1) Synthesis of 2-(2′,4′-difluoro-3′-trifluoromethylphenyl)-5-methylpyrimidine
[0085] The reaction formula is as follows:
[0086]
[0087] The specific steps are: under nitrogen atmosphere, 1.73g (10mmol) 2-bromo-5-methylpyrimidine, 2.48g (11mmol) 2,4-difluoro-3-trifluoromethylphenylboronic acid and 0.28g (0.4mmol) Dichlorobis(triphenylphosphine)palladium was dissolved in 50ml of DMF and stirred for 10min. Subsequently, 25 ml of an aqueous solution containing 3.18 g (30 mmol) of sodium carbonate was added dropwise to the reaction system. Stir the reaction under heating to 90°C for 8 hours. After the reaction solution was cooled to room temperature, it was extracted with dich...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com