Molecular malignancy in melanocytic lesions
A melanocytic, malignant technology, applied in the field of molecular malignancies in melanocytic lesions, can solve the problems of shortened life, inaccessible malignancies, appropriate treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0247] Detection can also be accomplished by visual comparison of the extent of enzymatic reaction of the substrate compared to a similarly prepared standard. Exemplary fluorescently labeled compounds include fluorescein isothiocyanate, rhodamine, phycoerythrin, phycocyanin, allophycocyanin, o-phthalaldehyde, Cy3, Cy5, Cy7, tetramethylrhodamine isothiocyanate , Phycoerythrin, Allophycocyanin, Texas Red and Fluorescamine. Fluorescent emitting metals such as 152 Eu, or another metal of the lanthanide series detectably labels the antibody. Other metal compounds that can be conjugated to the antibody include, but are not limited to, ferritin, colloidal gold such as colloidal superparamagnetic beads. These metals can be attached to the antibody using such metal chelating groups as diethylenetriaminepentaacetic acid (DTPA) or ethylenediaminetetraacetic acid (EDTA). The antibodies may also be detectably labeled by coupling them to chemiluminescent compounds. Examples of chemilumi...
Embodiment approach
[0337] The methods described herein can be implemented in a variety of ways, such as those involving classifiers. Several representative, non-limiting embodiments are described below.
[0338] In some method embodiments, gene expression data is input (e.g., manually or automatically) into a computer or other device, machine or device for application of the various algorithms described herein, when collecting and processing large numbers of gene expression data points This is particularly advantageous when . Other embodiments include the use of communication infrastructure, such as the Internet. Various forms of hardware, software, firmware, processors, or combinations thereof can be used to implement particular classifier and method embodiments. The software may be implemented as an application program tangibly embodied on a program storage device or as distinct portions of the software (e.g., as applets) implemented in the user's computing environment and in the inspector's...
Embodiment 1
[0403] Gene selection using discovery sets of clinically characterized skin samples
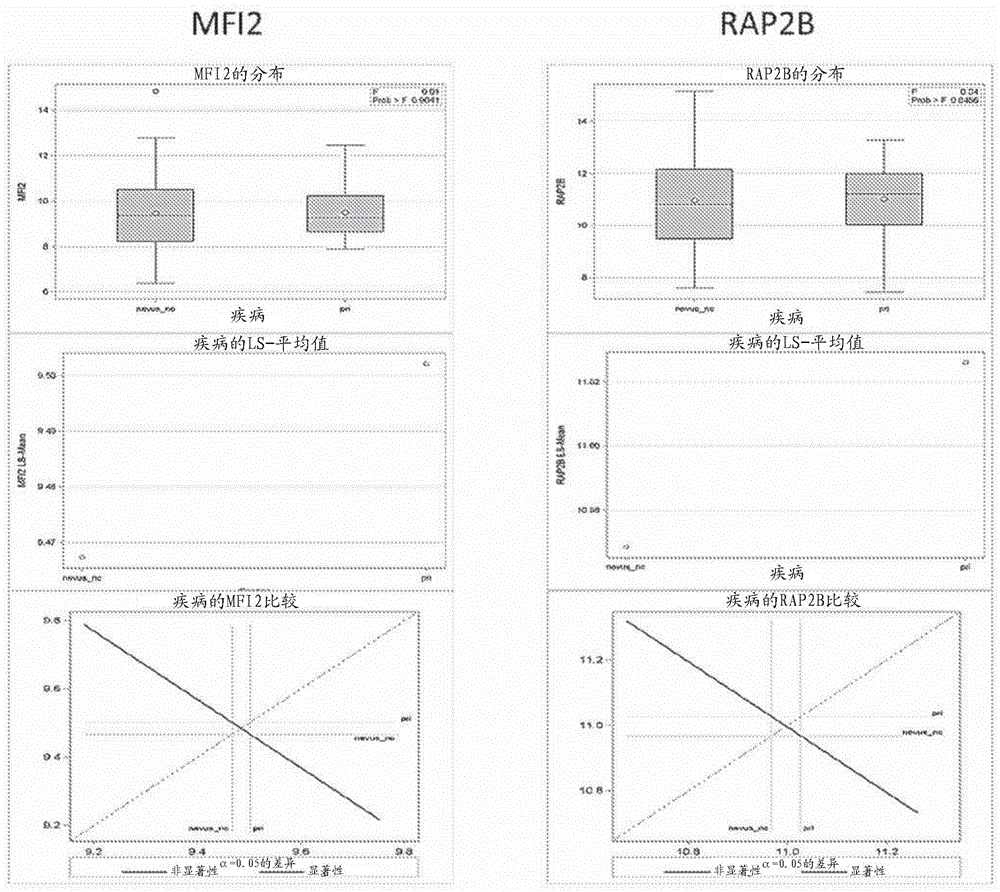
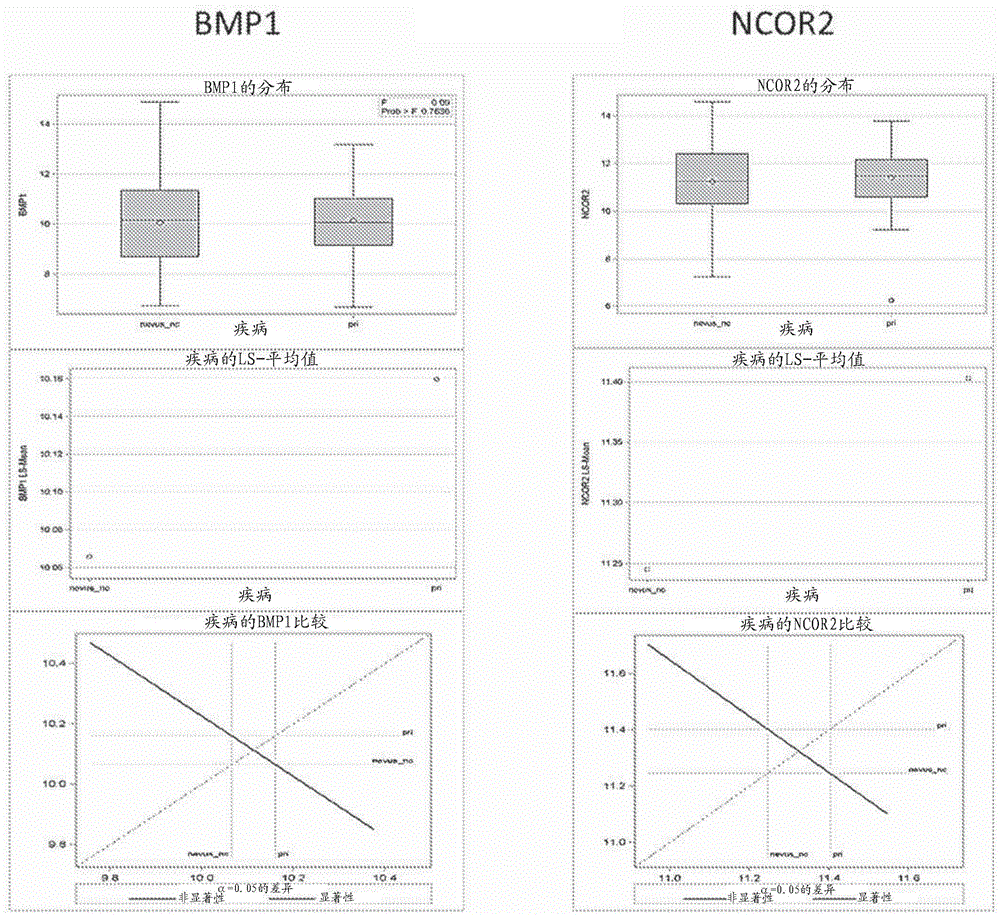
[0404] Nevus and melanoma cells, like all cells, also expressed a large number of genes, but most of these genes were not associated with these differences between the groups. Therefore, in order to extract useful gene information and reduce the scope, this example describes the initial screening of the expression of more than 2600 mRNA targets to identify the expression in formalin fixed and paraffin embedded (“FFPE”) from Significantly differentially expressed mRNAs in biopsied skin samples from human subjects. Details of the methods used throughout the examples are also described.
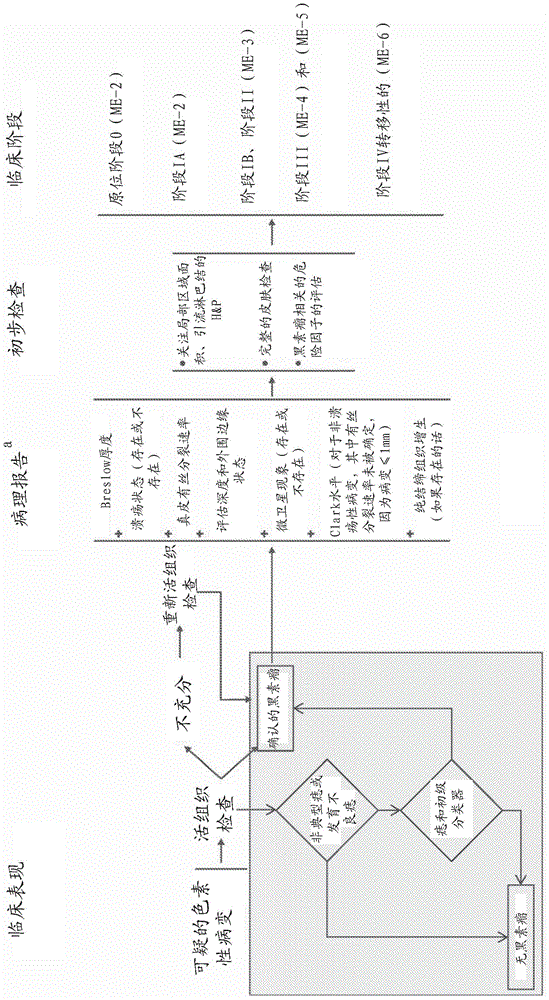
[0405] A discovery set of 39 FFPE tissue sections was provided by the John Wayne Cancer Institute Tissue Bank, each section approximately 5um thick, and mounted on glass slides. This set included 14 normal skin samples, 10 nevus samples, 5 primary melanoma samples, and 10 melanoma metastases samples.
[0406...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com