An orally disintegrating preparation
An oral disintegration and preparation technology, applied in the field of pharmaceutical preparations, can solve the problems of mad cow virus hidden dangers such as gelatin safety, high production costs, and hidden dangers, and achieve the effects of improving drug safety, taking convenience, and absorbing quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
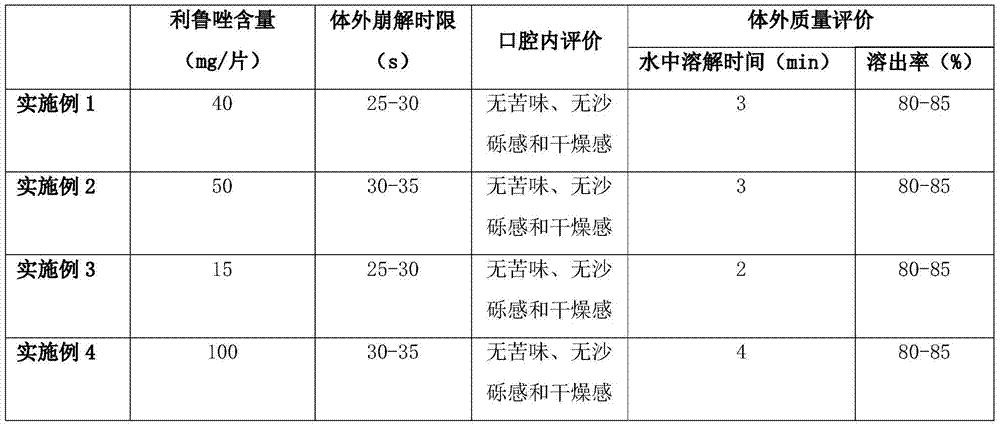
Examples
Embodiment 1
[0019] An orally disintegrating preparation provided by the invention comprises:
[0020] Therapeutic drugs 40g (8%)
[0021] Collagen Peptides 60g (12%)
[0022] Sugar alcohol 375g (75%)
[0023] Add other conventional auxiliary materials such as flavoring agents and lubricants in an appropriate amount. The above-mentioned percentages are percentages by weight, and the amount of 1000 preparations.
[0024] The orally disintegrating preparation of this embodiment is prepared by the following steps:
[0025] (1) Collagen peptide and sugar alcohol are dried and pulverized, and mixed through a 100-mesh sieve;
[0026] (2) Mix the medicine for treatment with the adjuvant of step (1) to prepare pellets by wet method, pass through a 40-mesh sieve, and mix uniformly;
[0027] (3) The mixture obtained in step (2) is directly compressed into tablets.
[0028] In this embodiment, the therapeutic drug is a benzothiazole drug, which is used for the treatment of patients with amyotro...
Embodiment 2
[0034] An orally disintegrating preparation provided by the invention comprises:
[0035] Drugs for treatment 50g (10%)
[0036] Collagen Peptides 90g(18%)
[0037] Sugar alcohol 300g (60%)
[0038] Others are the same as in Embodiment 1, and will not be repeated here.
Embodiment 3
[0040] An orally disintegrating preparation provided by the invention comprises:
[0041] Therapeutic drugs 15g (3%)
[0042] Collagen Peptides 25g (5%)
[0043] Sugar alcohol 400g (80%)
[0044] Others are the same as in Embodiment 1, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com