Catalyst used for alkane catalytic dehydrogenation and preparation method thereof
A technology for alkane dehydrogenation and catalysts, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve catalyst loss, low heat transfer efficiency, and catalyst environment Hazards have no hiding places and other issues, achieving the effect of high selectivity and high single-pass conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
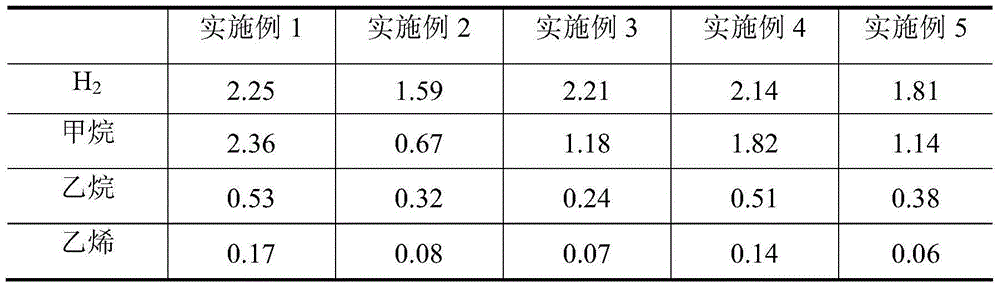
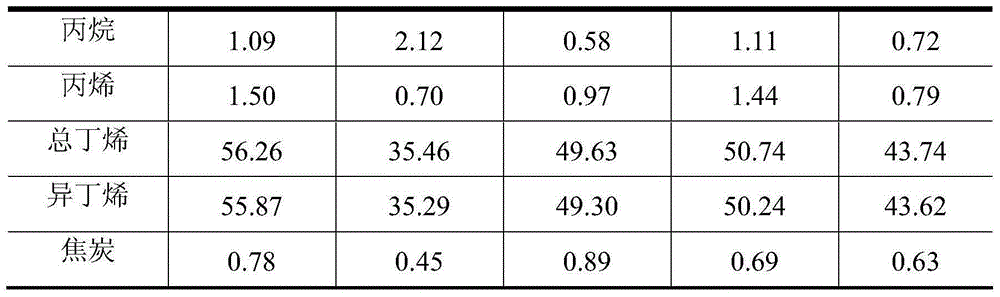
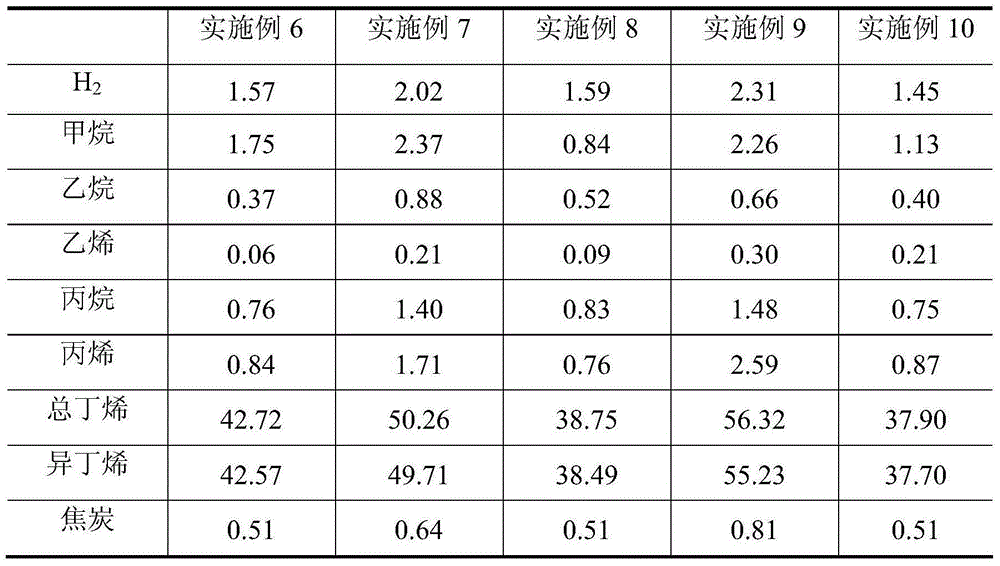
[0035] Weigh 73.08g of zinc nitrate (Zn(NO 3) 2 ·6H 2 O), dissolved in 50g of deionized water, completely dissolved, impregnated in 80g of silicon oxide (SiO 2 ), dried at 120°C for 6-8h, and then fired at 600°C for 5h. The catalyst evaluation results showed that the conversion rate of isobutane was 66.05wt%, the yield of isobutene was 55.87wt%, and the selectivity of isobutene was 84.59wt%.
Embodiment 2
[0037] Add 338.03 g of deionized water to 84.5 g of pseudo-boehmite, stir well in a water bath at 70° C., add hydrochloric acid to form a gel, and adjust the pH value to about 3-4. Take by weighing the magnesium nitrate (Mg(NO) of 144.45g 3 ) 2 ·6H 2 O) Add to the prepared gel, then add 50g of deionized water, mechanically stir evenly, dry at 120°C for 6-8h, then roast at 700°C for 8h, cool, crush and sieve, take 80-180 mesh The particles are ready for use as a carrier. 72.52g cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) dissolve in 50g deionized water and stir well. Co(NO) was impregnated on the prepared support 3 ) 2 Solution, dried at 140°C for 8-10h, then calcined at 700°C for 4h. The evaluation results of the catalyst showed that the conversion rate of isobutane was 41.91wt%, the yield of isobutene was 35.29wt%, and the selectivity of isobutene was 84.20wt%.
Embodiment 3
[0039] Add 338.03 g of deionized water to 84.5 g of pseudo-boehmite, stir well in a water bath at 70° C., add hydrochloric acid to form a gel, and adjust the pH value to about 3-4. Take by weighing the magnesium nitrate (Mg(NO) of 144.45g 3 ) 2 ·6H 2 O) Add to the prepared gel, then add 50g of deionized water, stir evenly, dry at 140°C for 6-8h, then roast at 700°C for 10h, cool and crush and sieve, take 80-180 mesh The particles are ready for use as a carrier. 73.08g zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) dissolve in 50g deionized water and stir well. Impregnated Zn(NO 3 ) 2 Solution, dried at 140°C for 6-8h, then calcined at 600°C for 5h. The evaluation results of the catalyst showed that the conversion rate of isobutane was 56.01wt%, the yield of isobutene was 49.30wt%, and the selectivity of isobutene was 88.02wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conversion rate | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Selectivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com