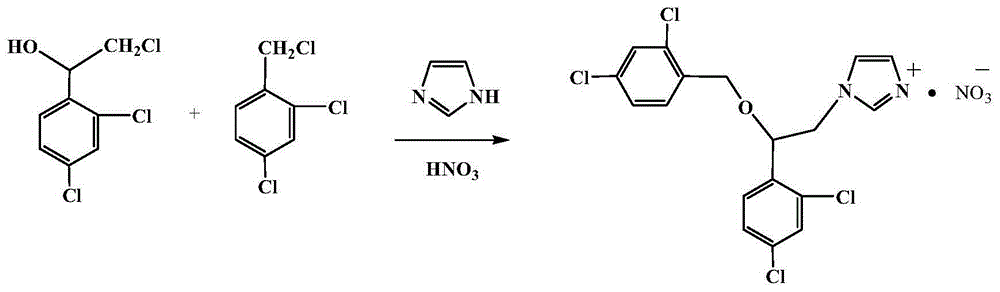
Preparation method of miconazole nitrate
A technology of miconazole nitrate and imidazole, applied in directions such as organic chemistry, can solve the problems of low yield, harsh conditions, expensive reagents and the like, and achieve the effects of high yield, safe reaction and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a 500 ml three-necked flask, 22.6 grams (0.1 moles) of 2,4-dichloro-2'-chlorophenylethanol, 8.2 grams (0.12 moles) of imidazole, and 23.5 grams of 2,4-dichlorobenzyl chloride ( 0.12 moles) and 163 grams of 1-methyl-3-butylimidazolium hexafluorophosphate ionic liquid were reacted at 50° C. for 5 hours. After the reaction was finished, the temperature of the reaction solution dropped to room temperature (25° C.), and after the pH value of the reaction body fluid was adjusted to 1 with an aqueous nitric acid solution with a volume concentration of 40%, the layers were left to stand, and the lower layer was taken to reclaim the ionic liquid. Concentrate under pressure until the solid precipitates, take the concentrate and let it cool to room temperature to obtain the crude product of miconazole nitrate salt, and finally recrystallize it with absolute ethanol to obtain 34 grams of pure white solid product with a yield of 71% (with 2,4-dichloro- 2′-Chlorophenethyl alcohol ...
Embodiment 2
[0019] In a 500 ml three-necked flask, 22.6 grams (0.1 mole) of 2,4-dichloro-2'-chlorophenylethanol, 6.8 grams (0.1 mole) of imidazole, and 19.6 grams of 2,4-dichlorobenzyl chloride ( 0.1 mole) and 245 grams of 1-methyl-3-butylimidazolium hexafluorophosphate ionic liquid were reacted at 10° C. for 10 hours. After the reaction is finished, the reaction liquid is lowered to room temperature, and the pH value of the reaction liquid is adjusted to 2 with an aqueous solution of nitric acid with a volume concentration of 50%, and the layers are left to stand, and the lower layer is taken to recover the ionic liquid, and the upper layer is concentrated under reduced pressure until solids are precipitated, and then placed Cool to room temperature to obtain the crude product of miconazole nitrate, and finally recrystallize with absolute ethanol to obtain 33 g of white solid pure product with a yield of 69%. HPLC analysis content is ≥ 99%. The melting point is 179-183°C.
Embodiment 3
[0021] In a 500 ml three-necked flask, 22.6 grams (0.1 moles) of 2,4-dichloro-2'-chlorophenylethanol, 10.2 grams (0.15 moles) of imidazole, and 29.3 grams of 2,4-dichlorobenzyl chloride ( 0.15 moles) and 496 grams of 1-methyl-3-butylimidazolium hexafluorophosphate ionic liquid were reacted at 100° C. for 1 hour. After the reaction, the reaction liquid was lowered to room temperature, and the pH value of the reaction liquid was adjusted to 3 with an aqueous nitric acid solution with a volume concentration of 15%, and the layers were allowed to stand, and the lower layer was taken to recover the ionic liquid, and the upper layer was concentrated under reduced pressure until solids were precipitated, and then placed Cool to room temperature to obtain the crude product of miconazole nitrate, and finally recrystallize with absolute ethanol to obtain 32.6 g of white solid pure product with a yield of 68%. HPLC analysis content is ≥ 99%. The melting point is 179-183°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com