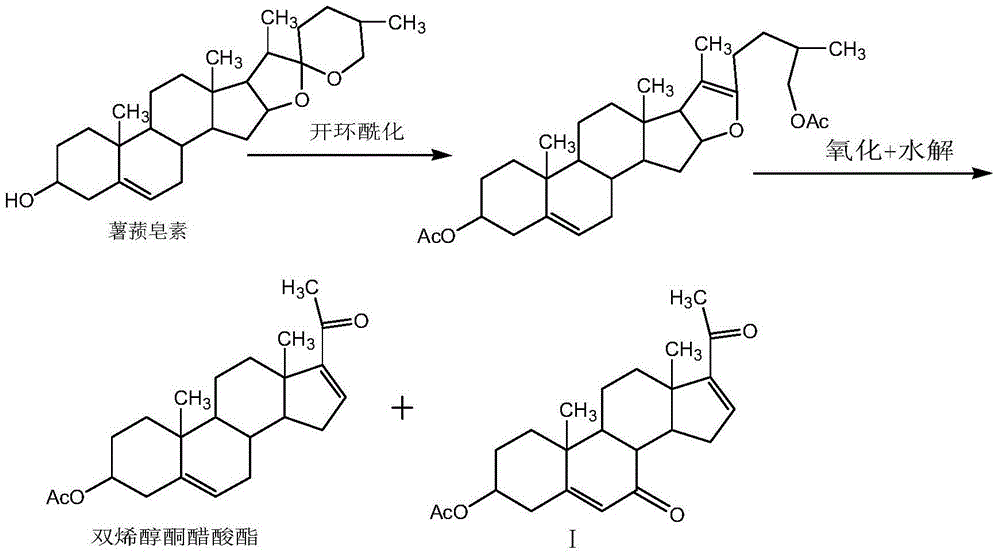
Synthesis method of main impurities of dehydropregnenolone acetate
A technology of dienolone acetate and diketene acetate is applied in the field of synthesis of main impurities of diketene acetate, can solve the problems of long time consumption, low purity and the like, and achieves a short synthesis time and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
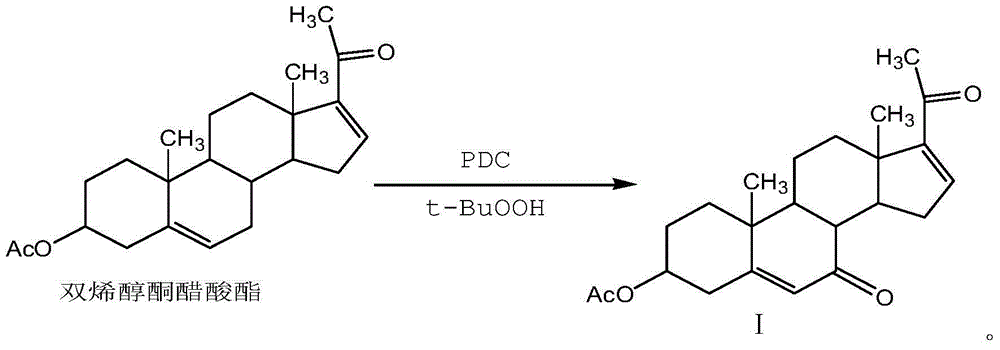
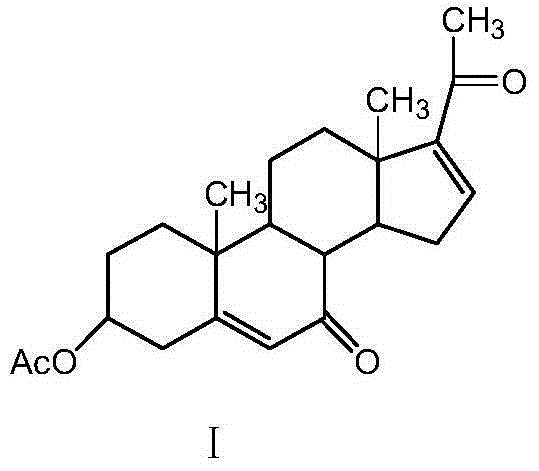
[0025] Add 200ml of benzene and 10.0g of dienolone acetate into the three-necked flask, add 50g of pyridinium dichromate, 15ml of tert-butanol peroxide, and 5.0g of diatomaceous earth under stirring, and stir for 2 hours at 20°C. Filter through a diatomaceous earth funnel, collect the filtrate, evaporate the filtrate under reduced pressure to remove the solvent, concentrate and dry the filtrate to obtain the crude product of compound I, add 5ml of dichloromethane to dissolve the crude product, and separate by chromatography column to obtain compound I, chemical name: 3β-acetoxy -pregna-5,16-diene-7,20-dione, the structure of which is shown in Formula I. The chromatographic column material is 200 mesh silica gel, and the eluent is: cyclohexane: ethyl acetate = 2:1 (V:V).
[0026]
[0027] It is a white powder product, 7.5 g, yield: 75%; HPLC: 98.2%.
[0028] MS (mz): 370 (M+).
[0029] 1 HNMR (CDCl 3 ): δ: 0.90 (3H, s, 18-CH 3 ), 1.20 (3H, s, 19-CH 3 ), 1.25, 1.9, 0 (e...
Embodiment 2
[0032] Add 500ml of benzene and 20.0g of dienolone acetate into the three-necked flask, add 140g of pyridinium dichromate, 34ml of tert-butanol peroxide, and 16.0g of diatomaceous earth under stirring, stir at 25°C for 3h, and use a Filter through a diatomaceous earth funnel, collect the filtrate, evaporate the filtrate under reduced pressure to remove the solvent, and dry the filtrate to obtain the crude product of compound I. Add 16ml of dichloromethane to dissolve the crude product, and separate by column chromatography to obtain compound I. The white powder product is 15.2g, and the yield is : 76%; HPLC: 98.5%. The chromatographic column material is silica gel, and the eluent is: cyclohexane:ethyl acetate=3:1 (V:V).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com