Preparation method and application of quinoxaline amide compound used as streptococcus mutans biological membrane inhibitor
A technology of Streptococcus mutans and quinoxaline imine, which is applied in the digestive system, antibacterial drugs, drug combinations, etc., can solve the problem that fluoride cannot be targeted, and inhibit the formation and maturation of Streptococcus mutans biofilm , simple structure, small molecular weight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation method of the culture of bacterial strain and liquid inhibitor
[0042] Preparation of test strains:
[0043] The test strain in this example involves Streptococcus mutans (strain number: 32401; obtained from China Medical Bacteria Collection and Management Center, website: http: / / www.cmccb.org.cn / ).
[0044] Components and preparation of medium:
[0045] Components of bovine heart and brain infusion (for convenience of description, hereinafter referred to as BHI) liquid medium and its preparation method: 37g of commercially available BHI powder (the BHI powder was purchased from Sigma, USA, item number: 53286) was added to 1000mL of distilled water Medium, high temperature and high pressure (121.3°C, 103.4kPa) sterilization for 15 minutes, cooled for later use. This liquid medium is a typical liquid medium for planktonic culture of Streptococcus mutans.
[0046] Components of BHI sucrose liquid medium and its preparation method: 37 g of...
Embodiment 2
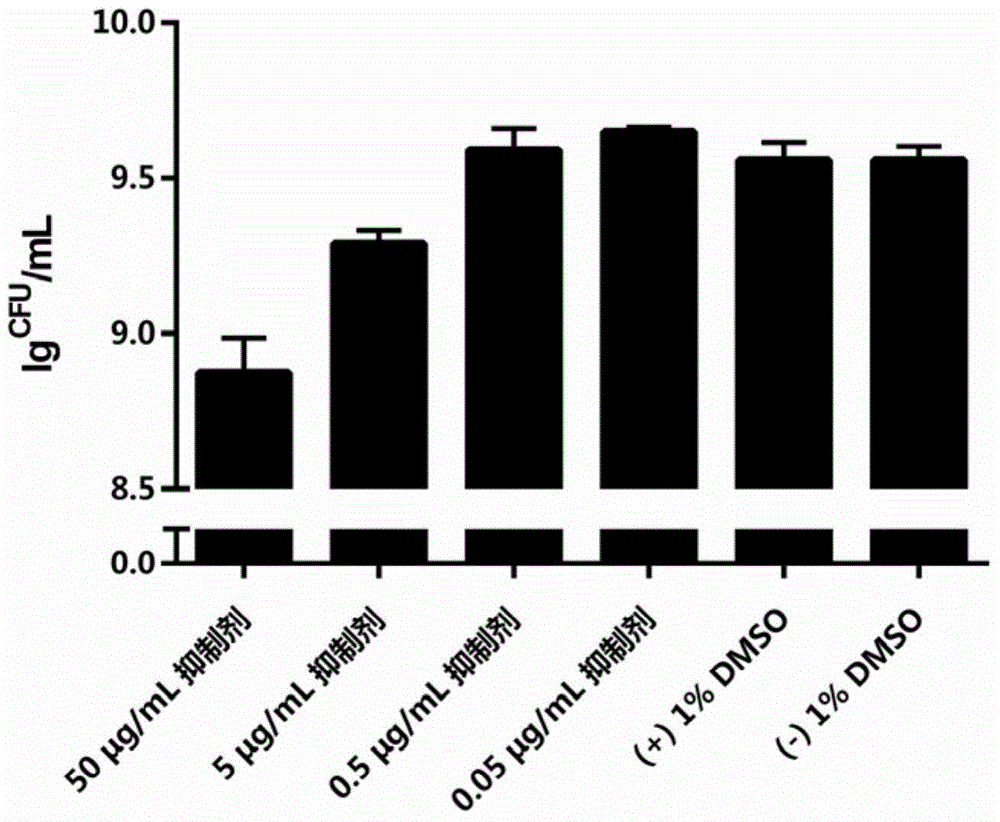
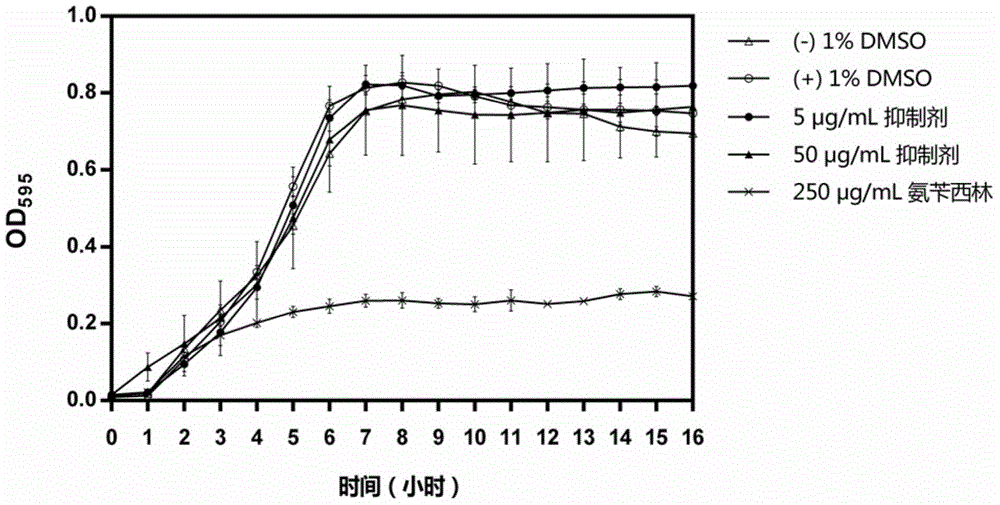
[0052] Embodiment 2: Inhibition experiment of inhibitor to mutant streptococcus biofilm formation
[0053] According to the aseptic procedure of Example 1, the Streptococcus mutans suspension (bacteria number: 32401) of Example 1 was inoculated respectively in BHI sucrose liquid medium containing different concentrations of inhibitors, to determine the inhibitory effect on Inhibitory activity of Streptococcus mutans biofilm formation. The specific method is: according to the ratio of 1:100, the Streptococcus mutans suspension in Example 1 was respectively inoculated into BHI with concentration gradients of 0.05 μg / mL, 0.5 μg / mL, 5 μg / mL and 50 μg / mL inhibitors. In sucrose liquid medium, at 37 °C, facultative anaerobic (5% CO 2 ) for 16 hours. Then, carefully suck out the planktonic bacteria and supernatant in the 48-well plate, and use pH=7.4, 0.01M phosphate buffer (the buffer was purchased from Thermo Company, USA, product number: P5368). Dissolve one bag of content in 10...
Embodiment 3
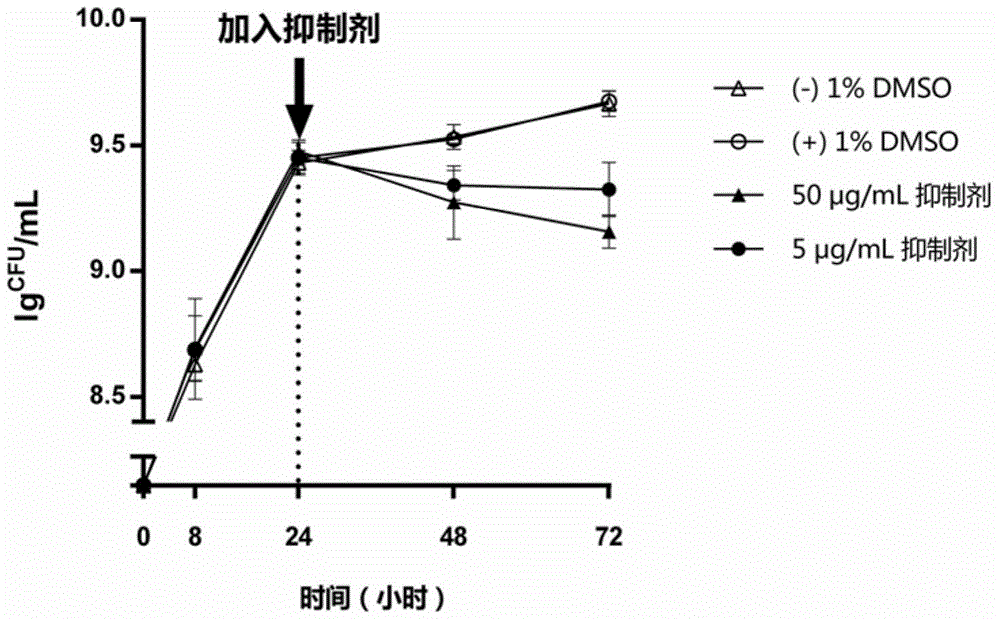
[0054] Embodiment 3: Inhibitor experiments on the inhibition of Streptococcus mutans biofilm maturation
[0055] According to the aseptic operation procedure of Example 1, first inoculate the Streptococcus mutans suspension (bacteria number: 32401) of Example 1 in BHI sucrose liquid medium to form a biofilm, and then add inhibitors of different concentrations respectively To determine the inhibitory activity of inhibitors on Streptococcus mutans biofilm maturation. The specific method is: inoculate the Streptococcus mutans suspension in Example 1 into a 48-well plate containing BHI sucrose liquid medium according to a dilution ratio of 1:100, and inoculate it at 37° C. under facultative anaerobic (5% CO 2 ) for 24 hours to form a biofilm. At this point, add inhibitors at final concentrations of 5 μg / mL and 50 μg / mL to the biofilm cultures of Streptococcus mutans, respectively, and incubate at 37°C in facultative anaerobic (5% CO 2) to continue culturing for 48 hours. Then, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com