Application of C-type lectin
A lectin, antibacterial technology, applied in the field of molecular biology, can solve the problem of no report of antiviral C-type lectin, and achieve the effect of improving the ability of antiviral and antibacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of C-type lectin recombinant protein
[0016] 1) Construction of plasmid pCTL1 expressing C-type lectin recombinant protein:
[0017] The C-type lectin gene sequence in the present invention has been reported (GenBank accession number XP_008329461.1). The cDNA of tongue sole was used as a template and PCR amplification was carried out with primers F1 and R1. The PCR conditions were: 94°C for 60s to pre-denature template DNA, then 94°C for 40s, 63°C for 60s, 65°C for 60s, 30 cycles. PCR products were purified with corresponding kits from Tiangen. The expression vector pET259 (see Hu YH, Zheng WW, Sun L. Identification and molecular analysis of a ferritin subunit from red drum (Sciaenops ocellatus) for the construction process of pET259. Fish Shellfish Immunol 2010; 28:678-86) with restriction endonuclease After digestion with enzyme SwaI, connect with the above purified PCR product with T4 DNA ligase, transform the connection solution into Escherichia coli...
Embodiment 2
[0025] Immunological application of recombinant C-type lectin
[0026] Step 1) Injection of lectin
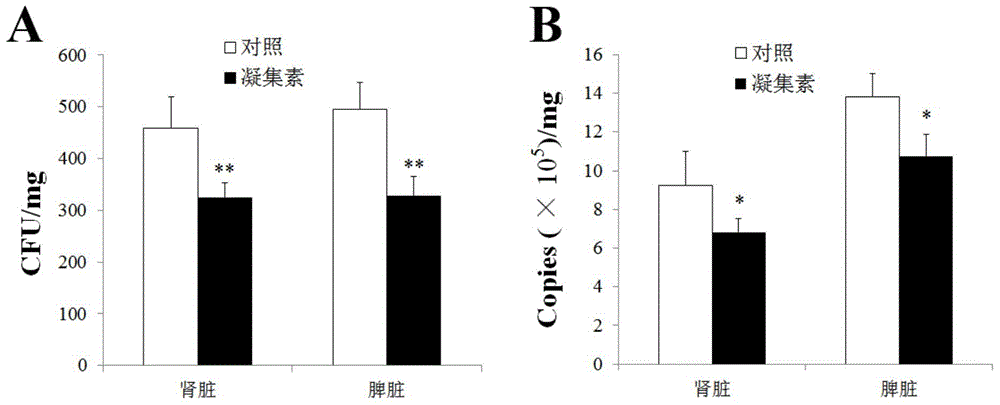
[0027] Dilute the C-type lectin obtained in Example 1 above to 20 ug / ml in PBS, which is the lectin dilution. Twenty tongue soles (about 10.1 g in weight) were randomly divided into 4 groups, 5 in each group. These 4 groups are named A, B, C and D respectively. Each fish in groups A and C was injected with 50ul of the lectin dilution, and each fish in groups B and D (control group) was injected with 50ul of PBS.
[0028] The composition of the PBS is by weight percentage: 0.8% NaCl, 0.02% KCl, 0.358% Na 2 HPO 4 .12H 2 O, 0.024% NaH 2 PO 4 , and the balance is water.
[0029] Step 2) Bacteria and virus suspension preparation
[0030] Cultivation of Vibrio anguillarum C312 to OD in LB medium 600 0.8, then centrifuged (5000g, 4°C, 10min), collected the bacteria, suspended in PBS to a final concentration of 10 6 cfu / ml is Vibrio eelium suspension. The cytomegalovirus RB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com