Impurity A in troxerutin and separation method thereof
A separation method and impurity technology, applied to the field of impurity A in troxerutin and its separation, capable of solving problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 : Separation of Impurity A from Troxerutin
[0048] Equipment: Agela Technologies CHEETAH HP-100
[0049] Chromatographic column: spherical silica gel, 15μm, 200mm×60mmID
[0050] Mobile phase: A isopropanol: B water = 50:1
[0051] Duty ratio: 100
[0052] Column pressure: 0.1MPa
[0053] Flow rate: 50ml
[0054] Detection wavelength: 254nm
[0055] Injection volume: 1g / injection
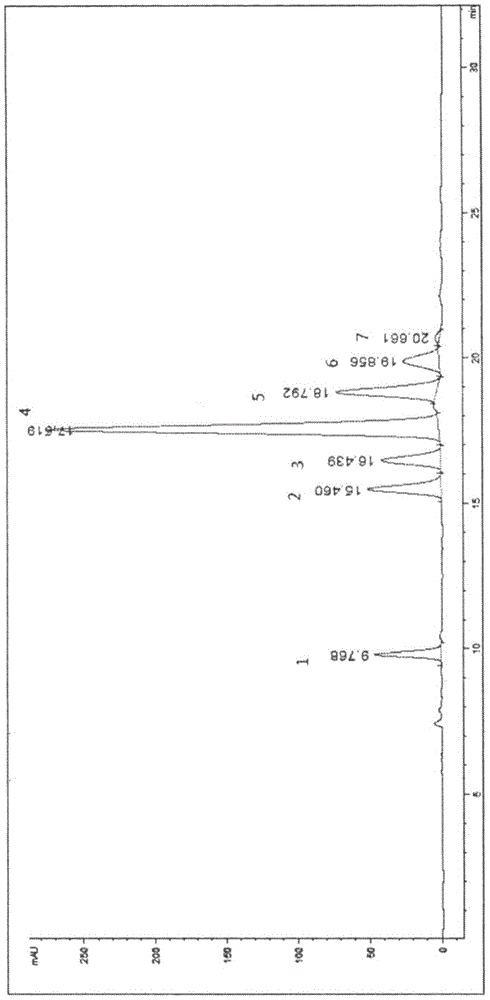
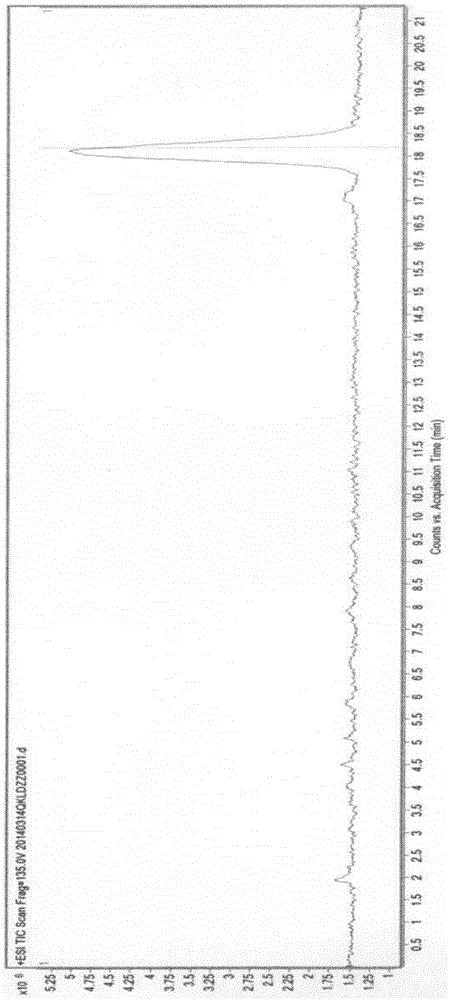
[0056] After dissolving troxerutin with the mobile phase, load it on the chromatographic column, and then use the mobile phase to elute and separate. After washing, the organic phase was concentrated to dryness under reduced pressure to obtain a yellow solid with a purity of 99%. Acid hydrolysis TLC detection, containing glucose and rhamnose. MS(ESI) calcd for [M+H] detected by mass spectrometry + :713.1, which is impurity A.
[0057] Separation of Narcisin from Rutin
[0058] Equipment: Agela Technologies CHEETAH HP-100
[0059] Chromatographic column: spherical ...
Embodiment 2
[0067] Embodiment 2: Narciscin in rutin produces impurity A
[0068] Equipment: Agela Technologies CHEETAH HP-100
[0069] Chromatographic column: spherical silica gel, 15μm, 200mm×60mmID
[0070] Mobile phase: A dichloromethane: B methanol = 200:1
[0071] Duty ratio: 150
[0072] Column pressure: 0.1MPa
[0073] Flow rate: 100ml
[0074] Detection wavelength: 254nm
[0075] Injection volume: 10g / injection
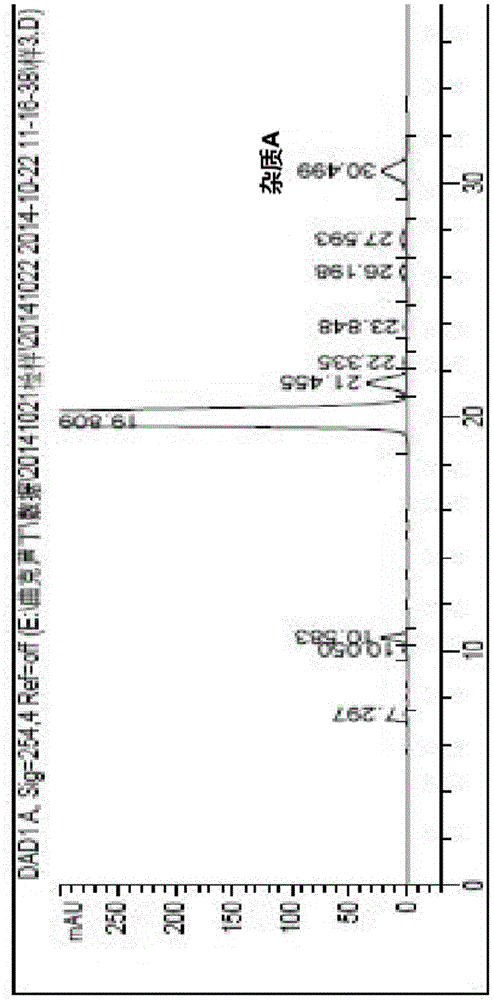
[0076] After dissolving the crude rutin with the mobile phase, load it on the chromatographic column, then elute and separate it with the mobile phase, collect the eluate in sections, and concentrate it to dryness under reduced pressure to obtain pure rutin with a purity of ≥98% and does not contain Narcisin.
[0077] Put 10.0 g of enriched pure rutin (purity ≥ 98%, does not contain narcissin) into a reaction vessel, add 40 ml of methanol, 0.1 g of sodium hydroxide, and 4 g of ethylene oxide, stir and heat to 100 ° C for reaction, 3 hours, troxerutin was obtaine...
Embodiment 3
[0087] Example 3 Mobile Phase Screening of Troxerutin Impurity A Separation Method
[0088] Equipment: Agela Technologies CHEETAH HP-100
[0089] Chromatographic column: spherical silica gel, 15μm, 200mm×60mmID
[0090] Duty ratio: 150
[0091] Column pressure: 0.1MPa
[0092] Flow rate: 50ml
[0093] Detection wavelength: 254nm
[0094] Injection volume: 1g / injection
[0095] Table 2 Screening of different mobile phases
[0096]
[0097] Troxerutin was dissolved in the mobile phase, loaded on the chromatographic column, and then eluted and separated with different mobile phases in Table 1. The eluate was collected in sections, concentrated under reduced pressure to dryness, and the concentrated solution was dissolved in dichloromethane. Washed with saturated citric acid, the organic phase was concentrated to dryness under reduced pressure to obtain a yellow solid.
[0098] It can be seen from the above experiments that the mobile phase is isopropanol: water, and w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com