Conductive polymer-graphene nanocomposite material, and preparation method and use thereof
A nano-composite material, conductive polymer technology, applied in the direction of improving process efficiency, can solve problems such as affecting the efficiency of adsorption and reduction of metal ions, adhesion, etc., to achieve good conductivity, increase specific surface area, and avoid agglomeration effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
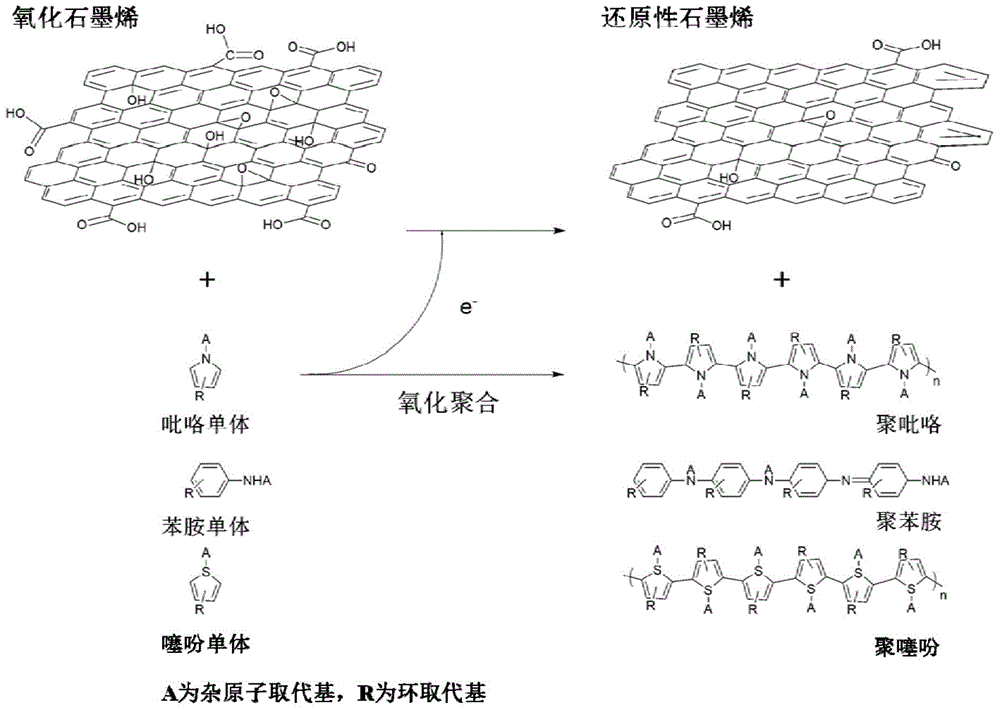
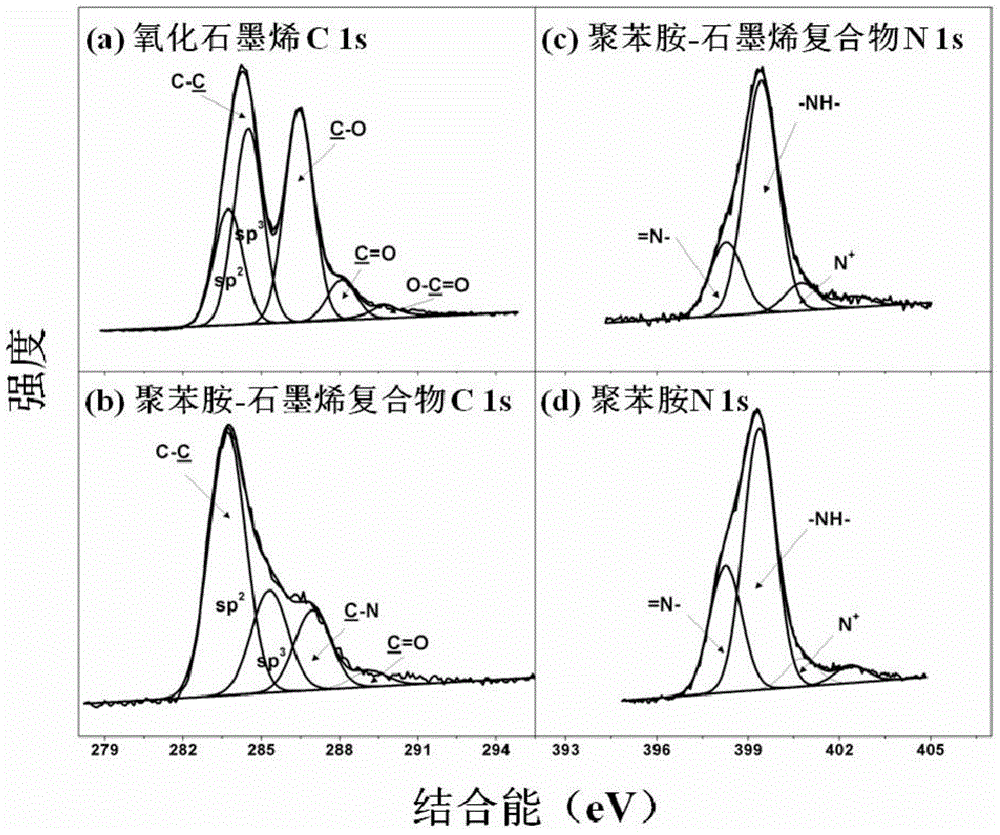
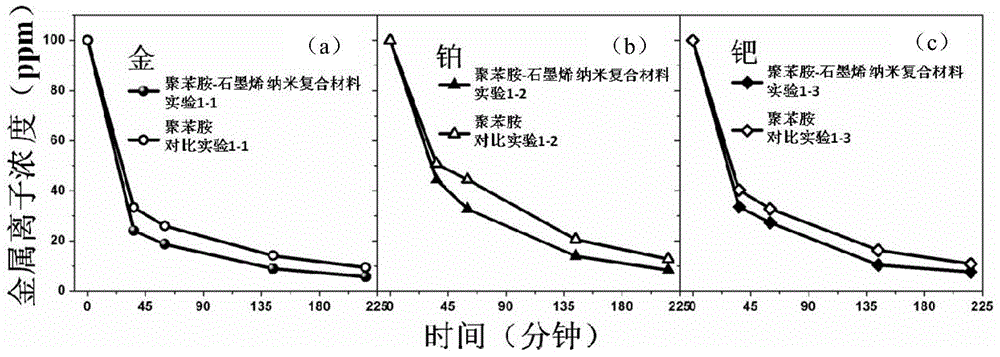
[0044] In this embodiment, the conductive polymer-graphene nanocomposite material is composed of polyaniline and graphene. The polyaniline uses graphene as a carrier and is dispersed on the surface of the graphene sheet structure to form the polyaniline-graphene nanocomposite material.
[0045] Preferably, the mass ratio of the conductive polymer monomer to graphene is 1:100 to 1:10.
[0046] The preparation method of the polyaniline-graphene nanocomposite material is as follows:
[0047] (1) Mix 1ml aniline monomer and 10ml dilute hydrochloric acid solution with a concentration of 1M thoroughly to obtain dilute aniline hydrochloric acid solution; disperse 100mg graphene oxide powder or fiber in 100ml deionized water, then add dilute aniline hydrochloric acid solution to it Stir uniformly, then vigorously stir at 70°C, react for 12 hours, and finally centrifuge the mixture at a high speed of 12000 rpm for 10 minutes to obtain a protonated polyaniline-graphene composite precipitate;
...
Embodiment 2
[0081] In this embodiment, the conductive polymer-graphene nanocomposite material is composed of polypyrrole and graphene. The polypyrrole uses graphene as a carrier and is dispersed on the surface of the graphene sheet structure to form the polypyrrole-graphene nanocomposite material.
[0082] Preferably, the mass ratio of the conductive polymer monomer to graphene is 1:100 to 1:10.
[0083] The preparation method of the polypyrrole-graphene nanocomposite material is as follows:
[0084] (1) Mix 1ml pyrrole monomer with 50ml dilute hydrochloric acid solution with a concentration of 1M to obtain dilute pyrrole hydrochloric acid solution; disperse 100mg graphene oxide powder or fiber in 500ml deionized water, then add dilute pyrrole hydrochloric acid solution to it Stir uniformly, then vigorously stir at 80°C and react for 6 hours. Finally, the mixture is separated by filtration to obtain the protonated polypyrrole-graphene composite precipitate;
[0085] (2) Deprotonate the protonated...
Embodiment 3
[0117] In this embodiment, the conductive polymer-graphene nanocomposite material is composed of polythiophene and graphene. The polythiophene uses graphene as a carrier and is dispersed on the surface of the graphene sheet structure to form the polythiophene-graphene nanocomposite material.
[0118] Preferably, the mass ratio of the conductive polymer monomer to graphene is 1:100 to 1:10.
[0119] The preparation method of the polythiophene-graphene nanocomposite material is as follows:
[0120] (1) Mix 1g of thiophene monomer with 100ml of 1M dilute hydrochloric acid solution to obtain dilute thiophene hydrochloric acid solution; disperse 100mg of graphene oxide powder or fiber in 1000ml of deionized water, and then add the dilute thiophene hydrochloric acid solution to it Stir uniformly, and then vigorously stir at 90°C. After reacting for 24 hours, the mixture is finally separated by filtration to obtain the protonated polythiophene-graphene composite precipitate;
[0121] (2) Dep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com