A kind of high-efficiency organic light-emitting material and its preparation and application
An electroluminescent material and organic technology, applied in the direction of luminescent materials, preparation of organic compounds, organic chemistry, etc., can solve the problems of complex synthesis methods, low brightness, low efficiency, and inability to fully satisfy full-color display, and achieve high luminescence efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
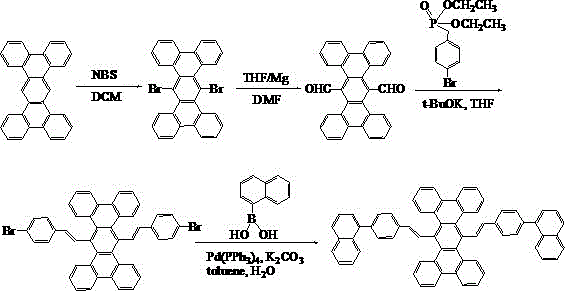
[0023] Embodiment 1: the synthesis of compound 001
[0024] The specific synthetic route is shown in the following formula:
[0025]
[0026] (1) Weigh 37.8g of tetrabenzotriphenyl, dissolve it in 200mL of dichloromethane, add 39.16g of NBS, raise the temperature to 40°C, stir and react for 24 hours, spin dry the organic solvent, and put the obtained crude product into the column layer Analysis (petroleum ether / dichloromethane=1:1) resulted in 41.83 g of light yellow solid 6,13-dibromotetrabenzotriphenyl.
[0027] (2) Under the condition of nitrogen protection, add 30ml of anhydrous tetrahydrofuran solution, 3.74g of magnesium bars, and 2 pieces of iodine into the three-necked flask. After the Grignard reagent triggers, add 41.83g of 6,13-dibromotetrabenzotriphenyl 100ml of anhydrous tetrahydrofuran solution, reacted for 3 hours under ice-water bath conditions, added dropwise 12ml of anhydrous N,N-dimethylformamide to the reaction solution, then slowly raised to room tempe...
Embodiment 2
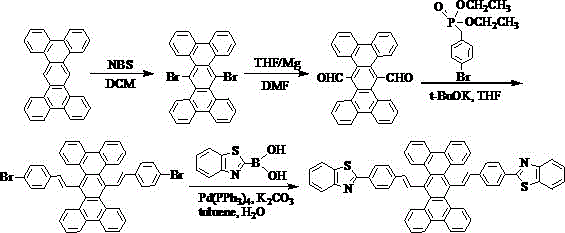
[0030] Embodiment 2: the synthesis of compound 002
[0031] The specific synthetic route is shown in the following formula:
[0032]
[0033] (1) Weigh 37.8g of tetrabenzotriphenyl, dissolve it in 200mL of dichloromethane, add 39.16g of NBS, raise the temperature to 40°C, stir and react for 24 hours, spin dry the organic solvent, and put the obtained crude product into the column layer Analysis (petroleum ether / dichloromethane=1:1) resulted in 41.83 g of light yellow solid 6,13-dibromotetrabenzotriphenyl.
[0034] (2) Under the condition of nitrogen protection, add 30ml of anhydrous tetrahydrofuran solution, 3.74g of magnesium bars, and 2 pieces of iodine into the three-necked flask. After the Grignard reagent triggers, add 41.83g of 6,13-dibromotetrabenzotriphenyl 100ml of anhydrous tetrahydrofuran solution, reacted for 3 hours under ice-water bath conditions, added dropwise 12ml of anhydrous N,N-dimethylformamide to the reaction solution, then slowly raised to room tempe...
Embodiment 3
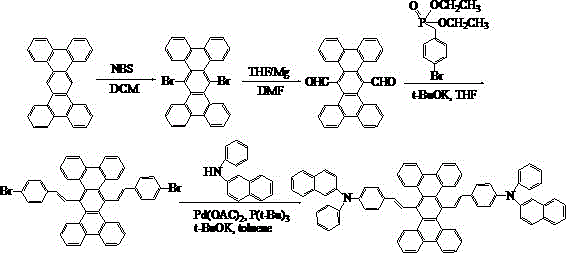
[0037] Embodiment 3: the synthesis of compound 003
[0038] The specific synthetic route is shown in the following formula:
[0039]
[0040] (1) Weigh 37.8g of tetrabenzotriphenyl, dissolve it in 200mL of dichloromethane, add 39.16g of NBS, raise the temperature to 40°C, stir and react for 24 hours, spin dry the organic solvent, and put the obtained crude product into the column layer Analysis (petroleum ether / dichloromethane=1:1) resulted in 41.83 g of light yellow solid 6,13-dibromotetrabenzotriphenyl.
[0041] (2) Under the condition of nitrogen protection, add 30ml of anhydrous tetrahydrofuran solution, 3.74g of magnesium bars, and 2 pieces of iodine into the three-necked flask. After the Grignard reagent triggers, add 41.83g of 6,13-dibromotetrabenzotriphenyl 100ml of anhydrous tetrahydrofuran solution, reacted for 3 hours under ice-water bath conditions, added dropwise 12ml of anhydrous N,N-dimethylformamide to the reaction solution, then slowly raised to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com