Capecitabine tablet
A capecitabine and tablet technology, which is applied in the field of medicine, can solve the problems of extended tablet disintegration time, difficulty in direct tablet compression, and poor fluidity of capecitabine, and achieve the effect of fast dissolution and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
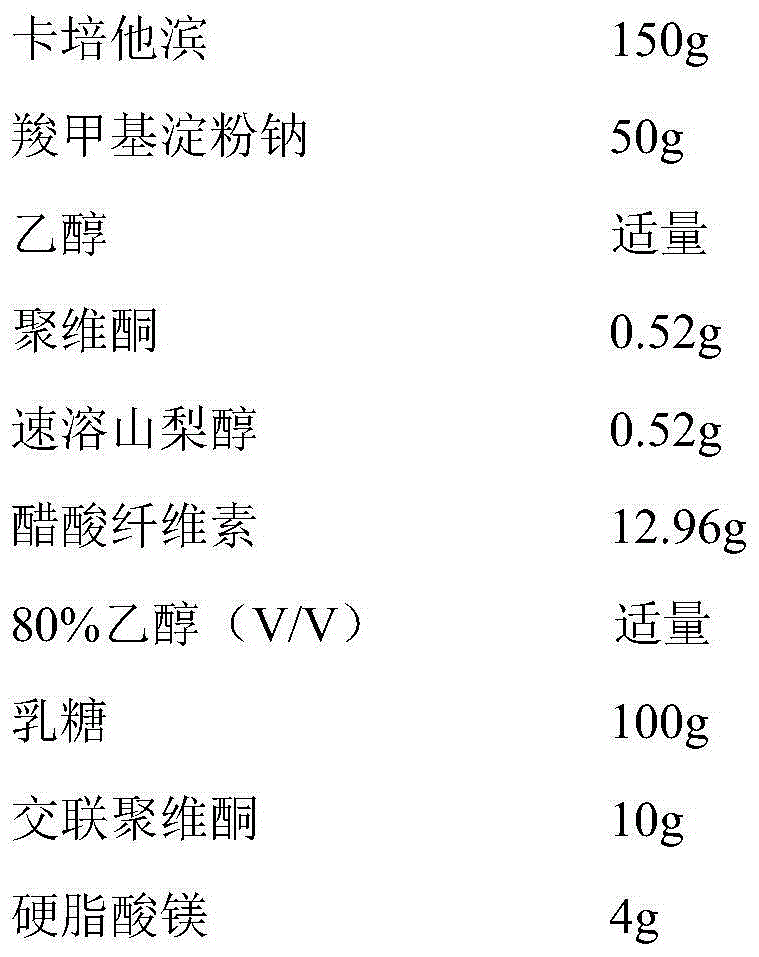
[0034]
[0035] Preparation Process
[0036] (1) Pass capecitabine through a 120-mesh sieve, mix evenly with the prescribed amount of carboxymethyl starch sodium, then add an appropriate amount of ethanol, granulate, granulate through a 16-mesh sieve, dry at 40°C, and set aside;
[0037] (2) The dried granules are added to the fluidized bed, coated with 80% ethanol (V / V) solution of cellulose acetate containing povidone and instant sorbitol, the coating temperature is 38°C, and the air inlet temperature is 42°C, spray speed 20ml / min;
[0038] (3) Mix the coated granules evenly with lactose, crospovidone and magnesium stearate, and compress into tablets.
Embodiment 2
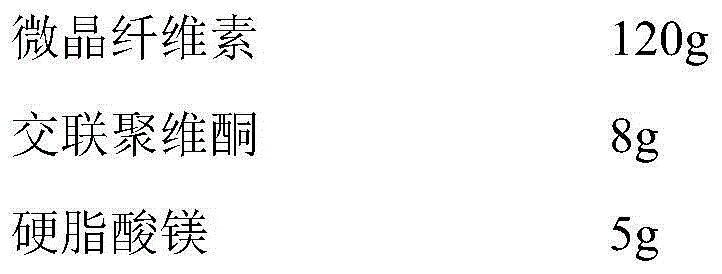
[0040]
[0041]
[0042] Preparation Process
[0043] (1) Pass the capecitabine through a 120-mesh sieve, mix evenly with the prescribed amount of crospovidone, then add an appropriate amount of ethanol, granulate, granulate through a 18-mesh sieve, dry at 45°C, and set aside;
[0044] (2) The dried granules are added to the fluidized bed, coated with 85% ethanol (V / V) solution of cellulose acetate containing povidone and instant sorbitol, the coating temperature is 40°C, and the air inlet temperature is 45°C, spray speed 25ml / min;
[0045] (3) Mix the coated granules with microcrystalline cellulose, crospovidone and magnesium stearate evenly, and compress into tablets.
Embodiment 3
[0047]
[0048] Preparation Process
[0049] (1) Pass the capecitabine through a 120-mesh sieve, mix evenly with the prescribed amount of crospovidone, then add an appropriate amount of ethanol, granulate, granulate through a 16-mesh sieve, dry at 40°C, and set aside;
[0050] (2) The dried granules are added to the fluidized bed, coated with 80% ethanol (V / V) solution of cellulose acetate containing povidone and instant sorbitol, the coating temperature is 38°C, and the air inlet temperature is 42°C, spray speed 20ml / min;
[0051] (3) Mix the coated granules evenly with lactose, crospovidone and magnesium stearate, and compress into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com