Method for acquiring lithium carbonate in zinnwaldite
A technology of iron lepidolite and lithium carbonate, which is applied in the direction of lithium carbonate; Effect of production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
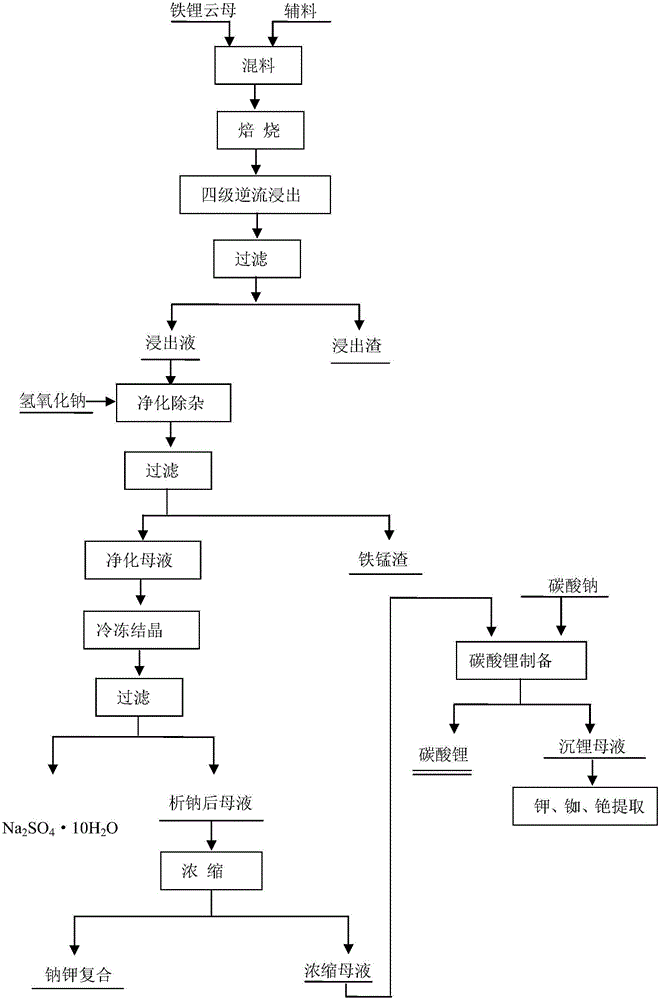
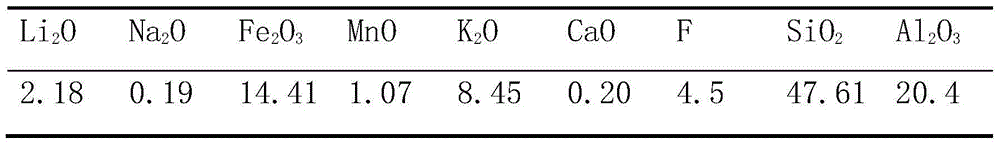
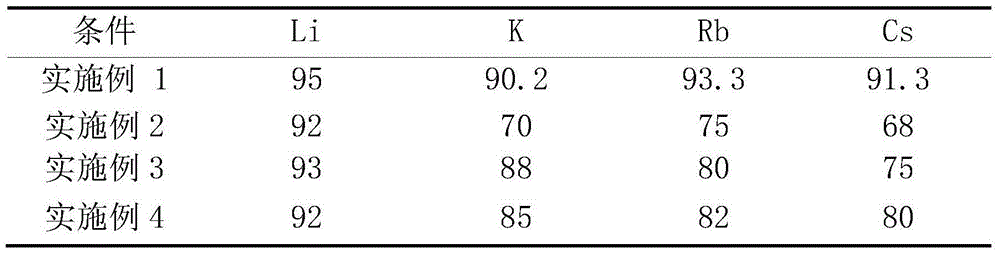
[0045] Get the iron lepidolite concentrate 1kg containing the main components in Table 1 and the roasting agent of 1.5Kg to carry out dry ball milling and mix, wherein roasting agent is made of Na 2 SO 4 , CaCl 2 , CaCO 3 and NaOH, the mass ratio of each component is 0.4:0.2:0.1:0.1; the mixed mixture is roasted at 850°C for 15 minutes to obtain clinker; the clinker is crushed and dissolved in a ball mill, and the clinker is crushed The particle size is 250 mesh, water is used as the leaching agent, the clinker and water are subjected to the first-stage leaching at a solid-to-liquid ratio of 0.5:1, the leaching temperature is 60°C, and the leaching time is 30 minutes; the fourth-stage countercurrent washing obtains the extract; The extract is filtered to obtain the leachate and leach residue, Li in the leachate 2 O concentration is about 20g / L. Then add Li in the leach solution 2 Stir with sodium hydroxide with an excess of 5% O content, purify and remove impurities, and ...
Embodiment 2
[0047] Get the roasting agent that contains the iron lepidolite concentrate 1kg and 1Kg of the main components in Table 1 to carry out dry ball milling and mix, wherein the roasting agent is made of Na 2 SO 4 , CaCl 2 , CaCO 3 and NaOH, the mass ratio of each component is 0.5:0.1:0.3:0.15; the mixed mixture is roasted at 900°C for 30 minutes to obtain clinker; the clinker is crushed and dissolved in a ball mill, and the clinker is crushed The particle size is 200 mesh, water is used as the leaching agent, clinker and water are subjected to the first-stage leaching according to the solid-to-liquid ratio of 2:1, the leaching temperature is 20°C, and the leaching time is 5 minutes; the fourth-stage countercurrent washing obtains the extract; The extract is filtered to obtain the leachate and leach residue, Li in the leachate 2 O concentration is about 10g / L. Then add Li in the leach solution 2 Stir with sodium hydroxide with an excess of 5% O content to purify and remove imp...
Embodiment 3
[0049] Get the iron lepidolite concentrate 1kg containing the main components in Table 1 and carry out the dry method ball milling mixing with 1Kg roasting agent, wherein roasting agent is made of Na 2 SO 4 , CaCl 2 , CaCO 3 Composed of NaOH and NaOH, the mass ratio of each component is 1:0.8:0.25:0.05; the mixed mixture is roasted at 1100°C for 15 minutes to obtain clinker; the clinker is crushed and dissolved in a ball mill, and the clinker is crushed The particle size is 220 mesh, water is used as the leaching agent, the clinker and water are subjected to the first-stage leaching at a solid-to-liquid ratio of 3:1, the leaching temperature is 90°C, and the leaching time is 80 minutes; the fourth-stage countercurrent washing is used to obtain the extract; The obtained extract is filtered to obtain the leachate and leach residue, and Li in the leachate 2 O concentration is about 5g / L. Then add Li in the leach solution 2 Stir with sodium hydroxide with an excess of 5% O co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com