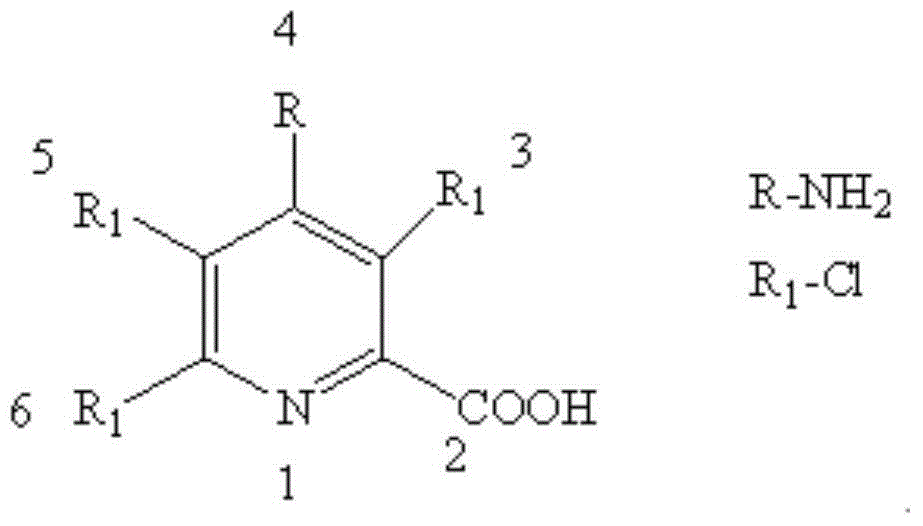
Preparation method of 3,4,5,6-tetrachloropyridine-2-carboxylic acid
A technology of clopicolinic acid and clopyralid, applied in the field of preparation of 3,4,5,6-tetraclopyralid, capable of solving problems such as environmental pollution and undiscovered preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
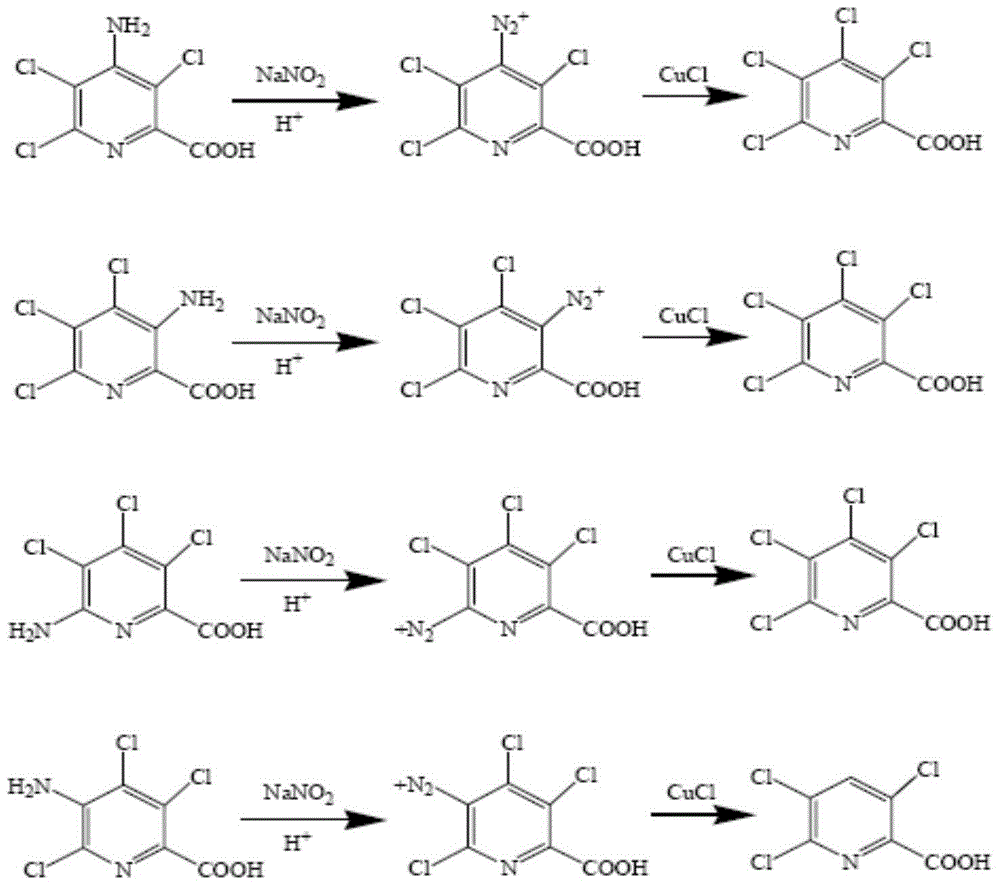
[0054] Preparation of 3,4,5,6-tetrachloropicolinic acid from 4-amino-3,5,6-trichloropicolinic acid (content above 95%)
[0055] Add 300g concentrated hydrochloric acid (36%, 2.95mol), 200g toluene, 40g 4-amino-3,5,6-tetrachloropicolinic acid (HPLC 98.5%, moisture<0.5%, 0.16mol) to the 1000ml flask successively, ice Water-cooled bath to between 0 ~ 5 ℃, stir evenly, quickly add 13.7g sodium nitrite (0.19mol), continue stirring for 30 minutes;
[0056] Below 5°C, add 19.0g of cuprous chloride (0.19mol), program the temperature rise, every 30 minutes to increase the temperature by 10°C, and continue to heat up to 50-55°C, HPLC analysis of 4-amino-3,5,6-tri When the content of clopicolinic acid is lower than 1.0%, the reaction ends.
[0057] Cool the water bath to below 30°C, then slowly add metered 40% potassium hydroxide solution dropwise to neutralize until the pH is between 8 and 9, filter, and the filter cake is washed with a small amount of water to precipitate copper hydro...
Embodiment 2
[0059] From the mixture of 4-amino-3,5,6-trichloropyridinecarboxylic acid ammonium salt and 6-amino-3,4,5-tetrachloropyridinecarboxylic acid ammonium salt (that is, the above residue I mainly contains 88%-92% 6-amino-3,4,5-trichloropicolinate ammonium salt, the rest is 4-amino-3,5,6-trichloroformic acid ammonium salt) to prepare 3,4,5,6-tetrachloropicolinic acid:
[0060] Prioritize drying the raw materials of the above residue I until the water content is <0.5% before the following experiments can be carried out.
[0061] Add 300g of concentrated hydrochloric acid (36%, 2.95mol), 200g of toluene, 40g of 4-amino-3,5,6-tetrachloropicolinate ammonium salt and 6-amino-3,4,5-tetrachloropyridine to a 1000ml flask successively Ammonium picolinate salt mixture (ammonium salt of 6-amino-3,5,6-triclopyridine is 85%, ammonium 4-amino-3,5,6-triclopyridine is 15%, 0.15 mol), ice water cooling bath to 15 ~ 25 ℃, stir evenly, quickly add 11.6g sodium nitrite (0.0.17mol), continue to stir f...
Embodiment 3
[0065] From 3-amino-4,5,6-trichloropicolinic acid, 4-amino-3,4,6-trichloropicolinic acid, 5-amino-3,4,6-trichloropicolinic acid and 6-amino- 3,4,5-Triclopyridine carboxylic acid mixture (that is, the above residue II, mainly contains 85%-90% of 3-amino-4,5,6-trichloroformic acid and 5-amino-3,4,6- A mixture of clopyralid, 6%-8% of 6-amino-3,4,5-trichloropicralic acid and the remainder 4-amino-3,5,6-trichloropicralic acid) to prepare 3,4,5 ,6-Tetrachloropicolinic acid:
[0066] Prioritize the drying treatment of the above-mentioned residue II raw materials, and the following experiments can only be carried out when the moisture content is <0.5%. Add 300g concentrated hydrochloric acid (36%, 2.95mol), 200g toluene, 40g 4-amino-3,5,6-tetrachloropicolinic acid, 3-amino-4,5,6-trichloropicolinic acid to a 1000ml flask in turn Mixture with 5-amino-3,4,6-tetrachloropicolinic acid (the ratio of 3-amino-4,5,6-trichloropicolinic acid to 5-amino-3,4,6-trichloropicolinic acid is 90 %, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com