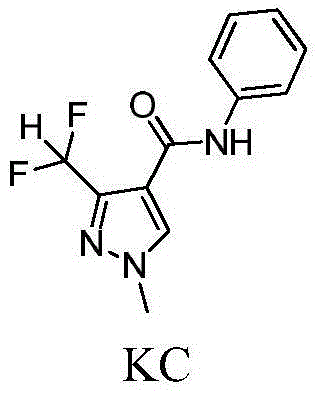
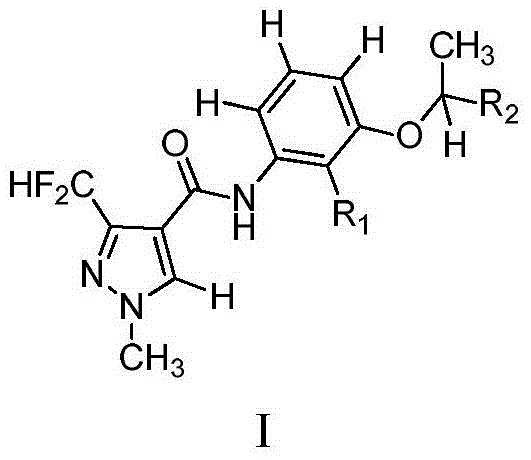
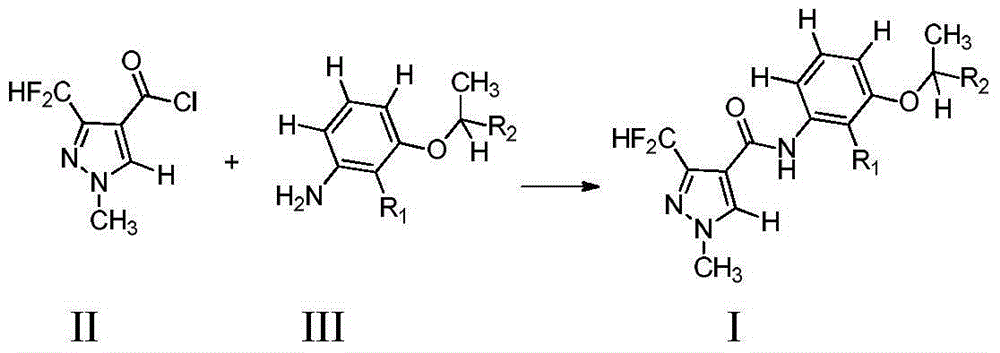
Pyrazole amide compound and application thereof
A technology of pyrazole amides and compounds, which is applied in the field of fungicides and can solve problems such as structural pyrazole amides that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0038] The following synthesis examples and biological activity measurement examples can be used to further illustrate the present invention, but are not meant to limit the present invention.
[0039] synthetic example
example 1
[0040] The preparation of example 1 compound 1:
[0041]
[0042] Add m-isopropoxyaniline (130 mg, 0.85 mmol, synthetic method refer to Bioorganic & Medicinal Chemistry, 2012, 20(3): 1213-1221), triethylamine (90 mg, 0.85 mmol) and 10 1 ml of dichloromethane, add 1-methyl-3-difluoromethylpyrazole-4-carbonyl chloride (170 mg, 0.85 mmol, synthetic method refer to example 6 in patent WO2008053043A1) dropwise under stirring at room temperature solution 10 ml. After dropping, the reaction was carried out at room temperature, and the reaction was completed after 3 hours. The reaction solution was poured into 30 ml of water, and the organic layer was taken. The organic layer was washed with saturated aqueous sodium bicarbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (eluent: ethyl acetate: petroleum ether = 1:2) to obtain 180 mg of compound 1 wit...
example 2
[0043] The preparation of example 2 compound 4:
[0044] (1) Synthesis of 1-(2-butoxy)-2-methyl-3-nitrobenzene
[0045]
[0046] To a DMF solution of 2-methyl-3-nitrophenol (2.00 g, 13.06 mmol) was added 2-bromo-sec-butane (2.14 g, 15.17 mmol) and potassium carbonate (2.16 g, 15.17 mmol), at The reaction was carried out at 90° C. for 2 hours. The reaction was stopped, ethyl acetate and water were added for extraction, the organic layer was washed with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was evaporated to dryness, and the residue was purified by column chromatography (ethyl acetate:petroleum ether=1:100) to obtain 1.65 g of solid.
[0047] (2) Synthesis of 2-methyl-3-(2-butoxy)aniline
[0048]
[0049] Add hydrazine hydrate (5.97 g, content: 40%, 47.76 mmol) to a solution of 1-(2-butoxy)-2-methyl-3-nitrobenzene (1.25 g, 5.97 mmol) in ethanol and 0.06 g of 10% palladium carbon, refluxed for two hours. Stop the reaction, filter, add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com