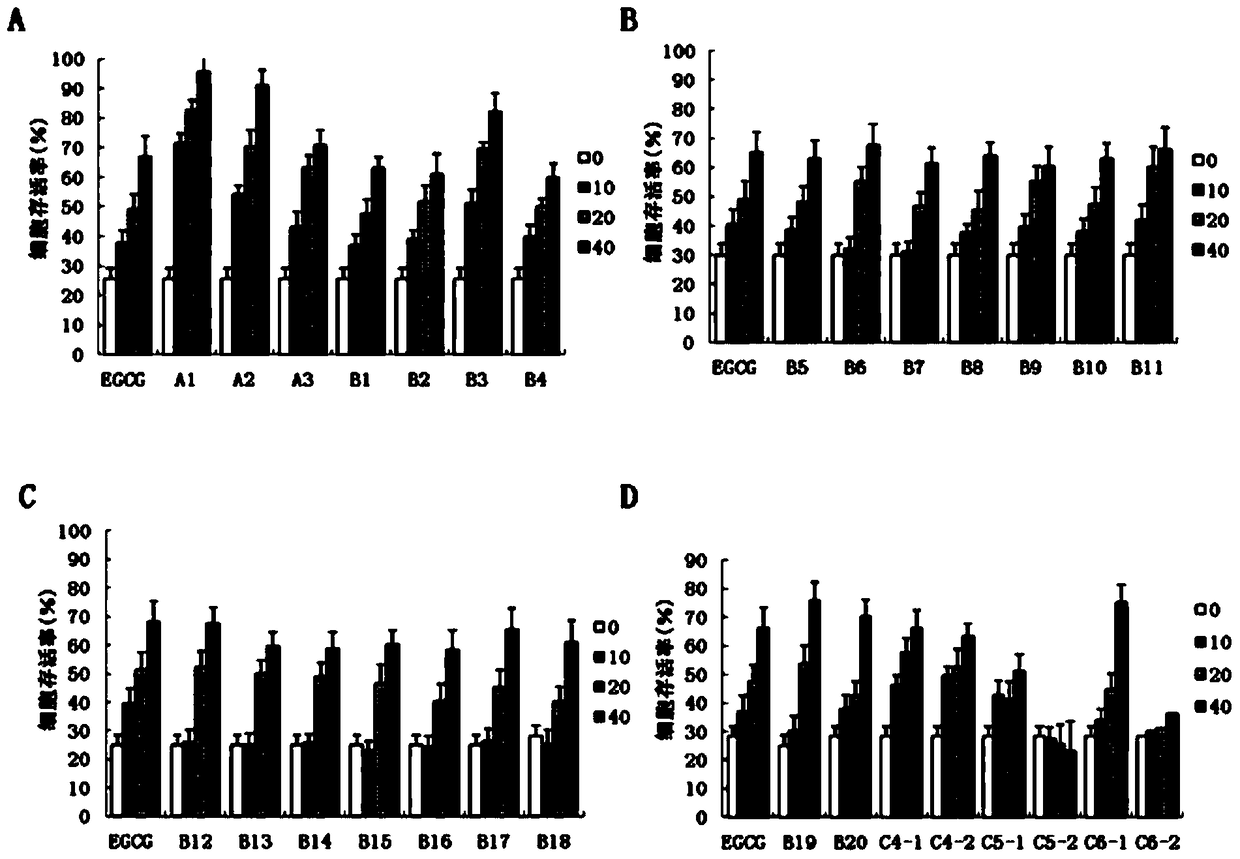
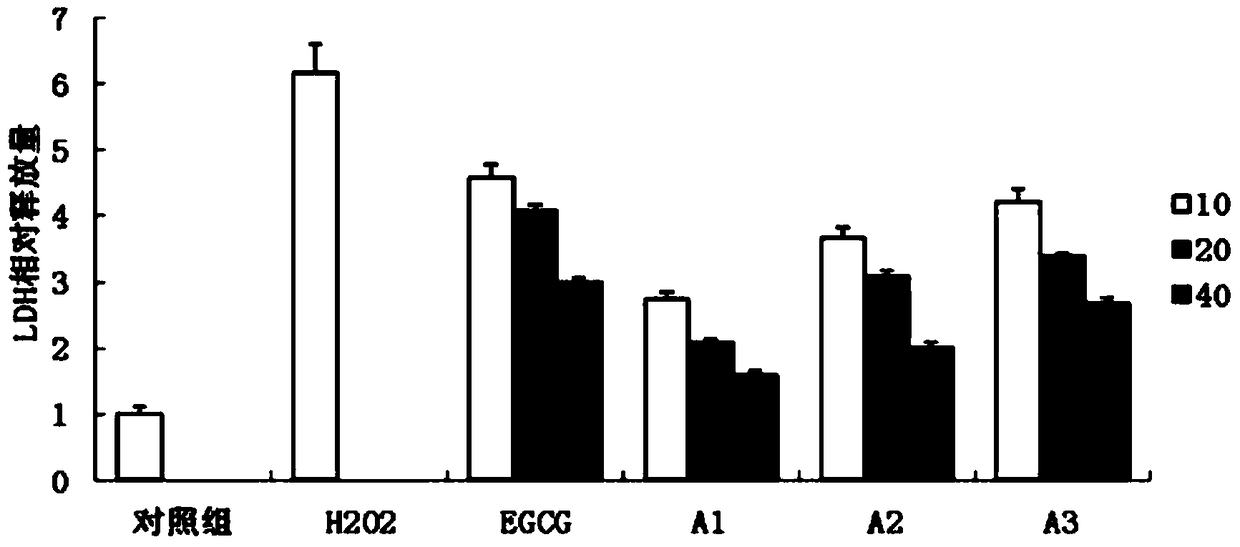
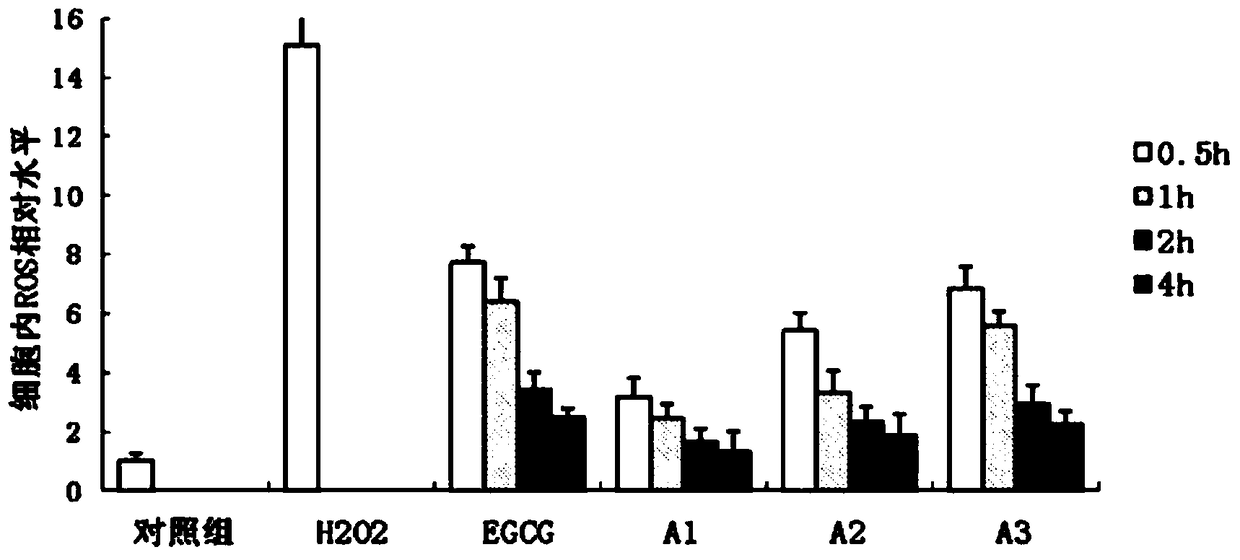
Preparation method of epigallocatechin gallate derivatives
A technology of epigallocatechin and gallate, applied in the field of compound application, can solve the problems of easy oxidation of hydroxyl group, difficult application, poor ability to penetrate cell membrane, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Synthesis of fully acylated EGCG
[0017]
[0018] 1. Compound A 1 (full acetylation) synthesis:
[0019] 115 mg of Compound 1 (EGCG) was dissolved in ethyl acetate, 200 mg of triethylamine was added, and then 250 mg of acetic anhydride was slowly added dropwise to react overnight. After the reaction is complete, use 10ml of water, 10ml of dilute hydrochloric acid, 10ml of saturated NaHCO 3 Wash the reaction solution 3 times, add anhydrous Na 2 SO 4 After drying, the solvent was recovered under reduced pressure, and recrystallized from ethanol to obtain 192 mg of white solid, yield: 95%.
[0020] 1 H NMR (500MHz, CDCl 3 )δ7.62(s,2H),7.23(s,2H),6.73(d,J=2.2Hz, 1H),6.60(d,J=2.3Hz,1H),5.63(d,J=1.5Hz, 1H), 5.17(s, 1H), 3.02(qd, J=17.9, 3.5Hz, 2H), 2.31–2.22(m, 24H).
[0021] 2. Compound A 2 (full propionylation) synthesis:
[0022] Procedure and Compound A 1 The synthesis is the same, except that acetic anhydride is replaced by propionic anhydride to obtain a ...
Embodiment 2
[0028] Synthesis of Partially Acylated EGCG
[0029]
[0030] Table 1: Conversion rates for the synthesis of partially acylated EGCG
[0031]
[0032]
[0033] The specific implementation process:
[0034] Dissolve 2g EGCG in ethyl acetate, add a certain amount of K 2 CO 3 Solid, then slowly add different equivalents of different acid chlorides (1-6 equivalents) dropwise, after 5 hours of reaction, TLC board monitors new spots, then use 20ml water, 20ml dilute hydrochloric acid, 20ml saturated NaHCO 3 Wash the reaction solution 3 times, anhydrous Na 2 SO 4 Dry, recover the solvent under reduced pressure, pass through a silica gel column, separate out different new spots, and use 1 HNMR normalized conversion.
Embodiment 3
[0036] Synthesis of Different Numbers of Hydroxyls and Different Numbers of Hydroxyl Peracetylation and Perpropionylation Based on EGCG Modification
[0037]
[0038] The specific implementation process:
[0039] 1. Synthesis of compound 4:
[0040] Compound 2 (7.24mmoL), KOH (15.93mmoL), and benzyl bromide (10.86mmoL) were dissolved in ethanol (20mL) and water (2.2mL), and heated to reflux for 20h. Then 20% KOH aqueous solution was added, the mixture was heated to reflux for 4 h, the reaction mixture was cooled, water was added, acidified with 2N hydrochloric acid, filtered with suction, and the obtained solid was recrystallized with ethanol to obtain compound 3, a white solid, with a yield of 70%-80%.
[0041] Compound 3-2 (R 2 =4-OBn): 1 H NMR (400MHz, DMSO) δ12.64(s, 1H), 7.90(d, J=8.8Hz, 2H), 7.40(ddd, J=23.7, 17.2, 7.2Hz, 5H), 7.10(d, J= 8.8Hz,2H), 5.18(s,2H).
[0042] Compound 3-3(R 3 =3,4-diOBn): 1 H NMR (400MHz, CDCl 3 )δ7.55(d, J=7.3Hz, 2H), 7.51–7.29(m, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com