Application of umbilical cord mesenchymal stem cells in the preparation of pharmaceutical preparations for the treatment of pulmonary interstitial fibrosis
A technology for pulmonary interstitial fibrosis and stem cells, which can be applied to animal cells, drug combinations, vertebrate cells, etc., can solve the problem of not obtaining positive results in the bleomycin lung injury model, and achieve low preparation cost and no treatment. Side effects, the effect of inhibiting the deposition of collagen fibers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
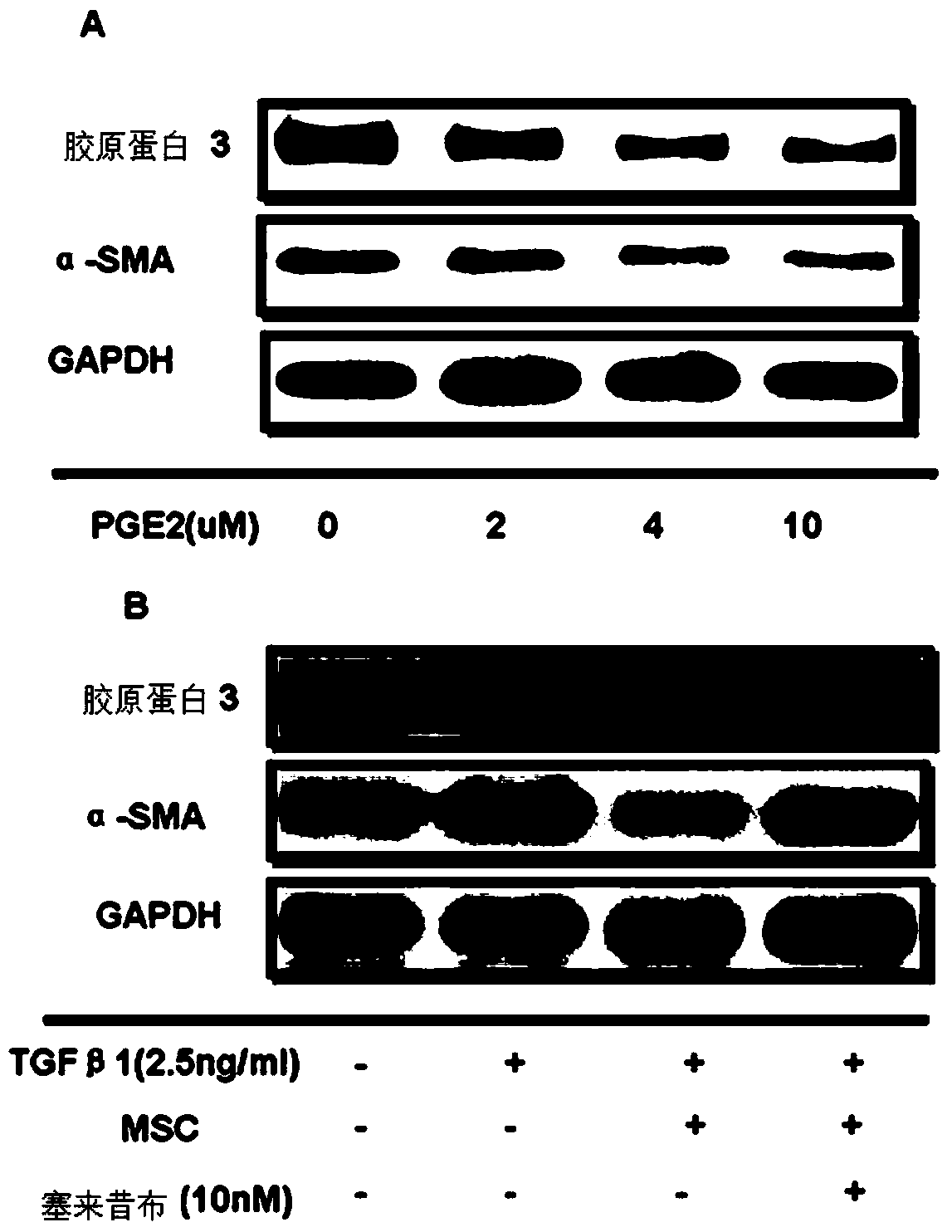
Image
Examples
Embodiment 1
[0034] Isolation, culture and identification of umbilical cord mesenchymal stem cells
[0035] 1 Isolation and culture of umbilical cord mesenchymal stem cells
[0036] The umbilical cord ligated after delivery of the fetus was taken, washed and disinfected, placed in umbilical cord preservation solution, and kept at a constant temperature of 6°C. Cut the Wharton's colloid to 2 mm with sterile tissue scissors 3 Add appropriate amount of medium to the tissue homogenate block, and place it in a carbon dioxide constant temperature and humidity incubator for culture. On the 16th day, when the area percentage of the cell clone reached 80%, it was digested and harvested to obtain P0 generation cells. Afterwards, digestion and passage were carried out, and the cells to be frozen were placed in a programmed cooling device, and the cells were lowered to below -80°C according to the standard freezing procedure.
[0037] 2 Identification of surface antigens of umbilical cord mesenchymal...
Embodiment 2
[0041] Effects of IFN-γ treatment on phenotype and secretion of cytokines and chemokines of umbilical cord mesenchymal stem cells
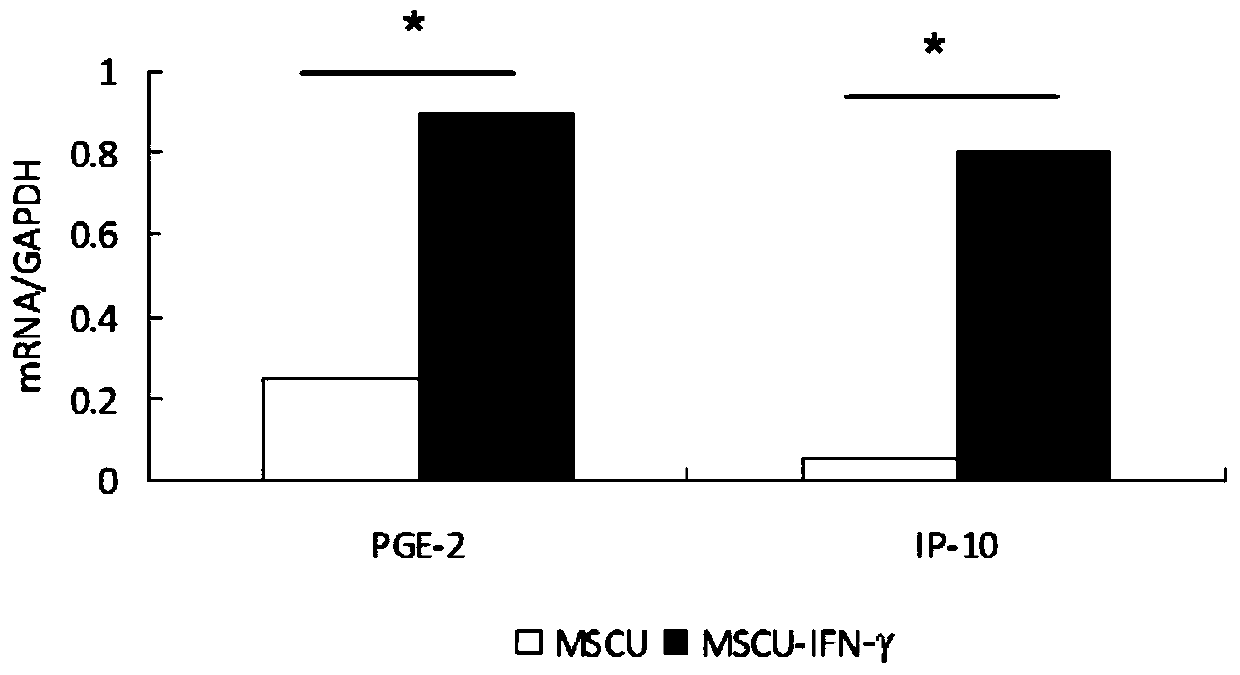
[0042] The P4 generation umbilical cord mesenchymal stem cells were mixed at 1×10 per well 5 Planted in a 6-well plate, changed to serum-free medium after 24 hours to starve the cells for 12 hours, added 20ng / ml recombinant human IFN-γ for 24 hours, collected the supernatant, and detected the anti-fibrosis effect by real-time fluorescent quantitative PCR The mRNA expression changes of cytokines and chemokines (IP-10, PGE2), the experimental results are shown in figure 1 , the cells were collected, and the changes of cell surface immune markers (CD90, CD29, CD73, CD105, CD45, CD34, CD19, CD14, HLA-DR) were detected by flow cytometry. The experimental results are shown in Table 1.
[0043] Table 1 The immunophenotype results of umbilical cord mesenchymal stem cells before and after IFN-γ pretreatment
[0044] cell surface immunolabeling ...
Embodiment 3
[0049] Preparation of culture supernatant of umbilical cord mesenchymal stem cells pretreated with IFN-γ and expression of cytokines, chemokines IP-10 and PGE2
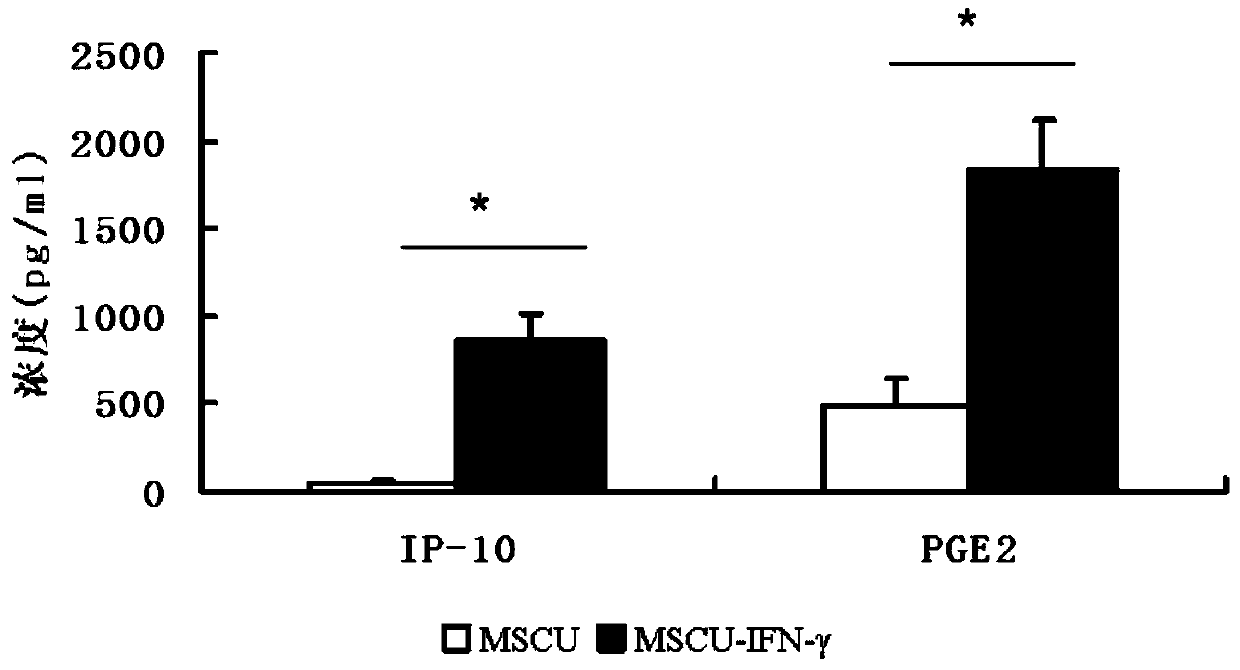
[0050] The mesenchymal stem cells were treated with 20ng / ml recombinant human IFN-γ for 24 hours, washed 3 times with PBS, added serum-free medium to continue culturing for 48 hours, and the culture supernatant was collected to be the supernatant required for subsequent animal experiments. The expression of IP-10 and PGE2 was detected by ELISA method, the experimental results are shown in figure 2 .
[0051] It was found that, if figure 2 As shown, in the culture supernatant of umbilical cord mesenchymal stem cells pretreated with IFN-γ, the levels of IP-10 and PGE2 were significantly higher than those of the untreated group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com