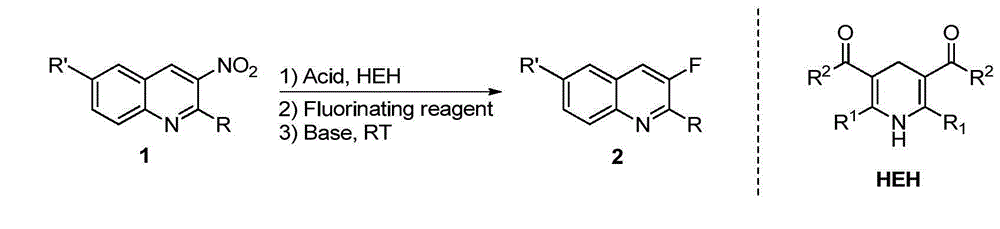
Method for synthesizing 3-quinoline derivatives
A technology of derivatives and fluoroquinoline, applied in the field of synthesizing 3-fluoroquinoline derivatives, can solve the problem of large amount of catalyst, and achieve the effects of convenient separation, mild reaction conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
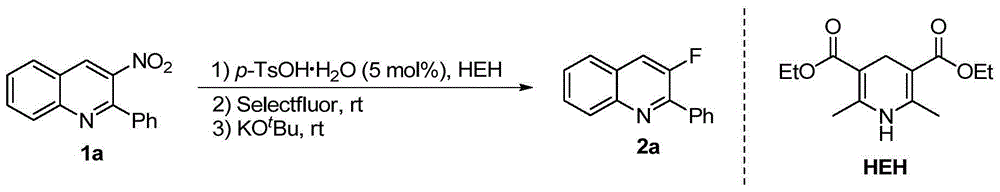
[0026] Embodiment 1: the synthesis of compound 2a
[0027] In a nitrogen-protected reaction flask, add 1a (0.2 mmol), p-toluenesulfonic acid monohydrate (0.01 mmol), HEH (0.22 mmol), and then add 3 ml of dry acetonitrile. After reacting at room temperature for 2 hours, Add Selectfluor (0.3 mmol) and continue the reaction for 5 minutes, then add potassium tert-butoxide (0.4 mmol) to the system, continue stirring at room temperature for 2 hours, remove the solvent under reduced pressure, and obtain the compound by silica gel column chromatography 2a, its isolated yield reached 94%. The reaction formula is as follows:
[0028]
[0029] 3-Fluoro-2-phenylquinoline(2a):94%yield,white solid,m.p.=36-38℃,R f =0.50(petroleum ether / EtOAc=20:1); 1 H NMR (400MHz, CDCl 3 )δ8.16(d,J=8.5Hz,1H),8.07(d,J=7.3Hz,2H),7.87-7.61(m,3H),7.56-7.44(m,4H); 13 C NMR (100MHz, CDCl 3 )δ155.2(d, 1 J F C=261.0Hz),149.2(d, 2 J FC =14.5Hz), 145.4(d, 5 J FC =2.9Hz), 135.9(d, 4 J FC =5.2Hz), 129....
Embodiment 2
[0031] The synthesis process of compounds 2b-2p is the same as that of Example 1, the difference from Example 1 is that only the substituents R and R' on the raw material 1a are different.
[0032] 3-Fluoro-2-o-tolylquinoline (2b): 95% yield, white solid, m.p.=69-71℃, R f =0.40(petroleum ether / EtOAc=20:1); 1 H NMR (400MHz, CDCl 3 )δ8.17(d,J=8.4Hz,1H),7.87-7.54(m,4H),7.46-7.30(m,4H),2.30(s,3H); 13 C NMR (100MHz, CDCl 3 )δ154.8(d, 1 J FC =257.8Hz), 151.7(d, 2 J FC =19.2Hz), 145.3(d, 4 J FC =2.9Hz),137.0,135.7(d, 4 J FC =3.6Hz),130.7,130.0(d, 5 J FC =1.6Hz),129.8,129.4,128.95(d, 5 J FC =2.5Hz), 128.66(d, 3 J FC =5.1Hz), 127.6, 127.15(d, 3 J FC =4.9Hz), 126.1, 119.2(d, 2 J FC =18.9Hz), 19.9(d, 4 J FC =2.9Hz); 19 F NMR (376MHz, CDCl 3 )δ-123.4(d,J=5.4Hz);HRMS(ESI)m / z Calculated for C 16 h 12 FN[M+H] + 238.1027, found 238.1034.
[0033] 3-Fluoro-2-m-tolylquinoline (2c):93%yield,colorless oil,R f =0.50(petroleum ether / EtOAc=20:1); 1 H NMR (400MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com