Acidic disinfectant and preparation method thereof
A disinfectant and acid technology, applied in the field of acid disinfectant and its preparation, can solve the problems of redox reaction, difficult to popularize, unstable content of effective peracetic acid and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
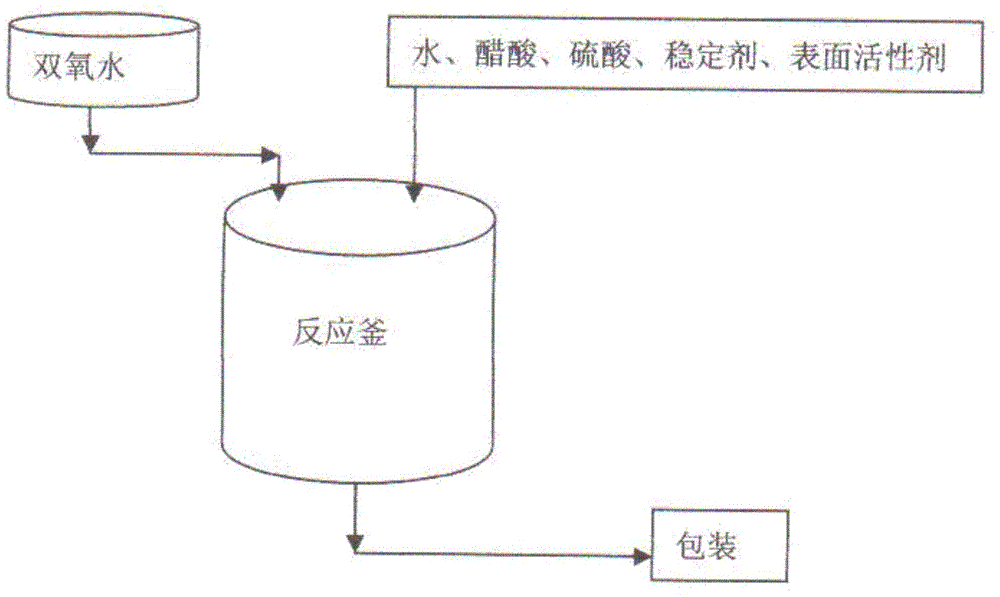
[0026]The weight composition distribution ratio of the disinfectant is 18 parts of water, 10 parts of acetic acid with a mass concentration of 90%, 72 parts of hydrogen peroxide with a mass concentration of 35%, 2 parts of sulfuric acid with a mass concentration of 90%, and 1 part of nonionic surfactant. 1 part, the nonionic surfactant is the ethylene oxide / propylene oxide block copolymer initiated by the fatty alcohol of C6-C12, 1 part of the stabilizer, and the stabilizer is composed of ethylenediaminetetraacetic acid, phosphoric acid Polyhydroxyl polycarboxylate multi-ligand complex formed after condensation reaction at a molar ratio of 1:1, 10 parts of C8-C16 alkylaryl sulfonic acid, 1 part of iminodisuccinic acid, sodium sulfate or carboxymethyl 1 part cellulose, 1 part dextrose pentaacetate, 1 part coconut oil hydroxyethyl imidazoline.
Embodiment 2
[0028] The weight composition distribution ratio of the disinfectant is 18 parts of water, 10 parts of acetic acid with a mass concentration of 90%, 72 parts of hydrogen peroxide with a mass concentration of 35%, 3 parts of sulfuric acid with a mass concentration of 90%, and 10 parts of a nonionic surfactant. part, the nonionic surfactant is the ethylene oxide / propylene oxide block copolymer initiated by the fatty alcohol of C6-C12, 10 parts of the stabilizer, and the stabilizer is composed of ethylenediaminetetraacetic acid, phosphoric acid Polyhydroxyl polycarboxylate multi-ligand complex formed after condensation reaction at a molar ratio of 1:1, 20 parts of C8-C16 alkylaryl sulfonic acid, 10 parts of imino disuccinic acid, sodium sulfate or carboxymethyl Cellulose 5 parts, dextrose pentaacetate 10 parts, coconut oil hydroxyethyl imidazoline 8 parts.
Embodiment 3
[0030] The weight composition distribution ratio of the disinfectant is 18 parts of water, 10 parts of acetic acid with a mass concentration of 90%, 72 parts of hydrogen peroxide with a mass concentration of 35%, 2 parts of sulfuric acid with a mass concentration of 90%, and 5 parts of nonionic surfactant. part, the nonionic surfactant is the ethylene oxide / propylene oxide block copolymer initiated by the fatty alcohol of C6-C12, 5 parts of the stabilizer, and the stabilizer is composed of ethylenediaminetetraacetic acid, phosphoric acid Polyhydroxy polycarboxylate multi-ligand complex formed after condensation reaction at a molar ratio of 1:1, 15 parts of C8-C16 alkylaryl sulfonic acid, 5 parts of imino disuccinic acid, sodium sulfate or carboxymethyl Cellulose 2 parts, dextrose pentaacetate 5 parts, coconut oil hydroxyethyl imidazoline 4 parts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com