Albendazole suspension and preparation method thereof
A technology of albendazole and suspension, which is applied in the field of pharmaceutical preparations, can solve the problems of fetal absorption and bone deformity, low solubility of albendazole, low bioavailability, etc., and achieves improved bioavailability and simple preparation process Feasible, good redispersibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The step of the preparation method of described a kind of albendazole suspension is:
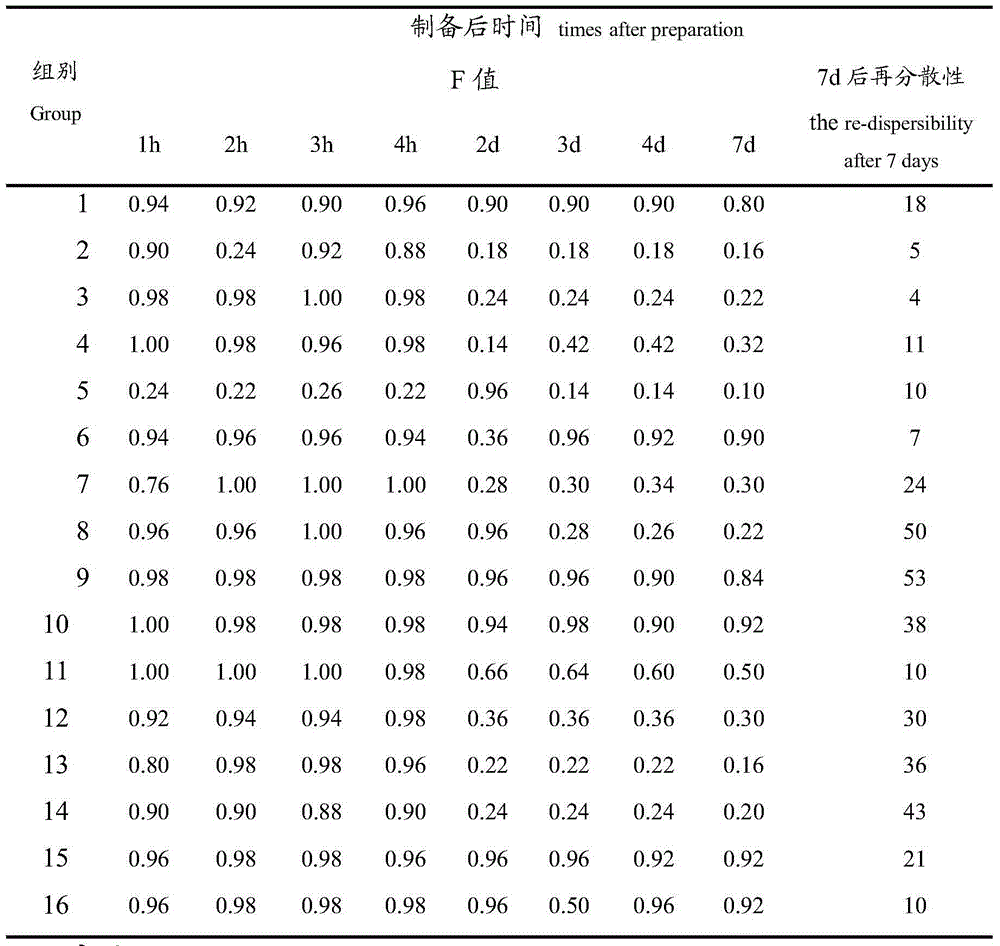
[0026] 1) Prescription screening: Carboxymethylcellulose sodium was determined as suspending agent, polysorbate-80 as wetting agent, citric acid as flocculant, and sodium benzoate as antioxidant through single factor test; then L16 (4 5 ) Orthogonal method is designed to screen the concentration of sodium carboxymethylcellulose, polysorbate-80, citric acid and sodium benzoate, and the physical properties of the suspension, sedimentation volume ratio, redispersibility, and drug content Using the chemical characteristics as an index, compare the stability of each prescription to determine the best prescription;
[0027] 2) Preparation: adopt the dispersion method, prepare the suspension with a high-speed homogeneous disperser, the steps are as follows:
[0028] a. Dissolve the suspending agent, flocculant and antioxidant in distilled water, and wait for swelling or dissolving for later...
Embodiment 1
[0043] A kind of albendazole suspension, prescription consists of:
[0044] 0.25g albendazole, 0.025g sodium carboxymethylcellulose, 0.0125mL polysorbate-80, 0.125g citric acid, 0.005g sodium benzoate, purified water to 25mL.
[0045] Its preparation method is:
[0046] (1) Dissolve the suspending agent, wetting agent, flocculant, and antioxidant in an appropriate amount of distilled water, and wait for swelling or dissolving for later use;
[0047] (2) Dissolve the wetting agent in a small amount of distilled water, then add albendazole for wetting, mix with a high-speed homogeneous disperser, transfer to a 25mL graduated cylinder, use a distilled water homogenizer several times, and transfer to the graduated cylinder;
[0048](3) Add distilled water to make up to 25mL, seal the mouth of the cylinder, mix well, and get ready.
Embodiment 2
[0050] A kind of albendazole suspension, prescription consists of:
[0051] 0.25g albendazole, 0.125g sodium carboxymethylcellulose, 0.25mL polysorbate-80, 0.2g citric acid, 0.075g sodium benzoate, purified water to 25mL.
[0052] Its preparation method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com