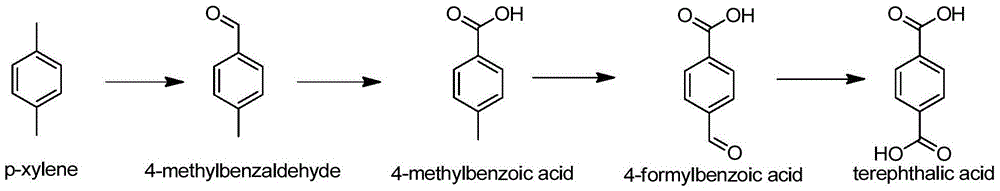
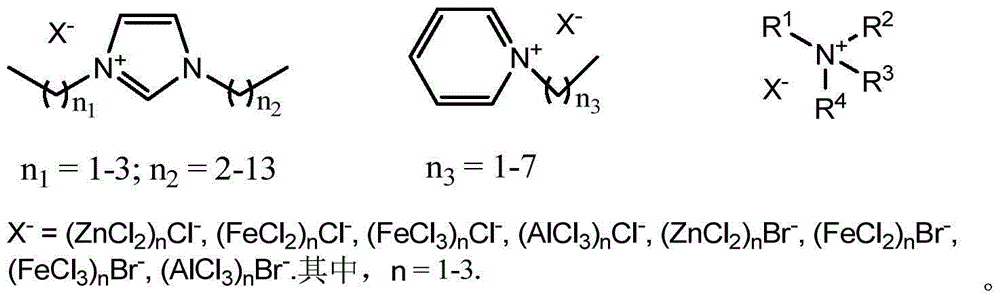Method for preparing 4-methylbenzaldehyde from isoprene and acrolein
A technology of p-tolualdehyde and isoprene, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as harsh reaction conditions, long process flow, and serious environmental pollution. Achieve the effects of mild reaction conditions, simple process operation, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of graphite oxide adopts the following methods (Marcano, DC; Kosynkin, DV; Berlin, JM; Sinitskii, A.; Sun, ZZ; Slesarev, A.; Alemany, LB; Lu, W.; Tour, JMACS Nano2010, 4 ,4806): Add 3g graphite and 1.5g sodium nitrate to 69ml98% concentrated sulfuric acid under 0℃ ice bath, stir and mix well; then gradually add 9g potassium permanganate, keep stirring, keep the temperature below 20℃; Stir in a 35℃ water bath for 7 hours; continue to slowly add 12g potassium permanganate and stir for 12 hours; after the reaction is cooled, pour it into 400ml ice water and stir; add 3mL30% H2O2 and stir; then add 400mL30%HCl and stir At least 30min; Finally, the product is filtered and washed with deionized water until the pH value is about 7, and the product is vacuum dried to obtain graphite oxide.
Embodiment 2
[0029] Weigh a certain amount of metal halide and stir and dissolve it in 10 mmol ionic liquid 1-butyl-3-methylimidazolium chloride (BmimCl) at 120°C. After 10 minutes of reaction, the Lewis acidic ionic liquid can be prepared in different proportions. Diels-Alder reaction.
Embodiment 3
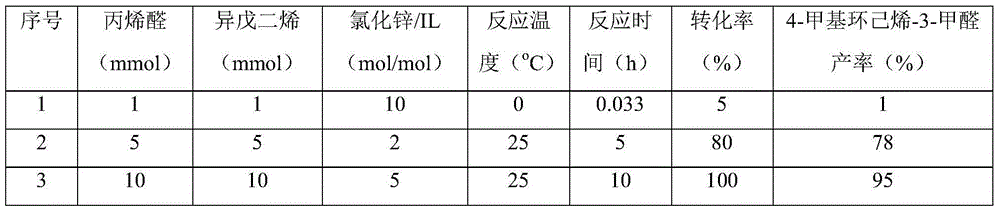
[0031] Add a certain amount of acrolein, isoprene and 10mmol BmimCl-ZnCl 2 (Mole ratio is 1:2) Put it into the flask, stir the reaction at different temperatures, after a certain period of time, sample and add about 1ml tetrahydrofuran to dilute, mix and centrifuge, and the product is qualitatively analyzed by GC-MS and quantitatively analyzed by GC external standard method. The results of the reaction are shown in Table 1.
[0032] Table 1. Diels-Alder reaction results in zinc chloride ionic liquid
[0033]
[0034]
[0035] The above results show that in the temperature range of 0℃-150℃, acrolein / isoprene / zinc chloride ionic liquid=1:1:10-1:1:1 (mol / mol / mol) in the reaction solution , Can obtain 4-methylcyclohexene-3-carbaldehyde, the yield is between 1%-95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com