1H-indazole-3-aminobiphenyl compound as well as preparation method and application thereof
An aminobiphenyl and compound technology, applied in the field of biomedicine, can solve the problems of decreased drug efficacy, affecting the quality of life and lifespan of patients, etc., and achieve the effects of good inhibitory activity, good application prospect and scientific research value, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
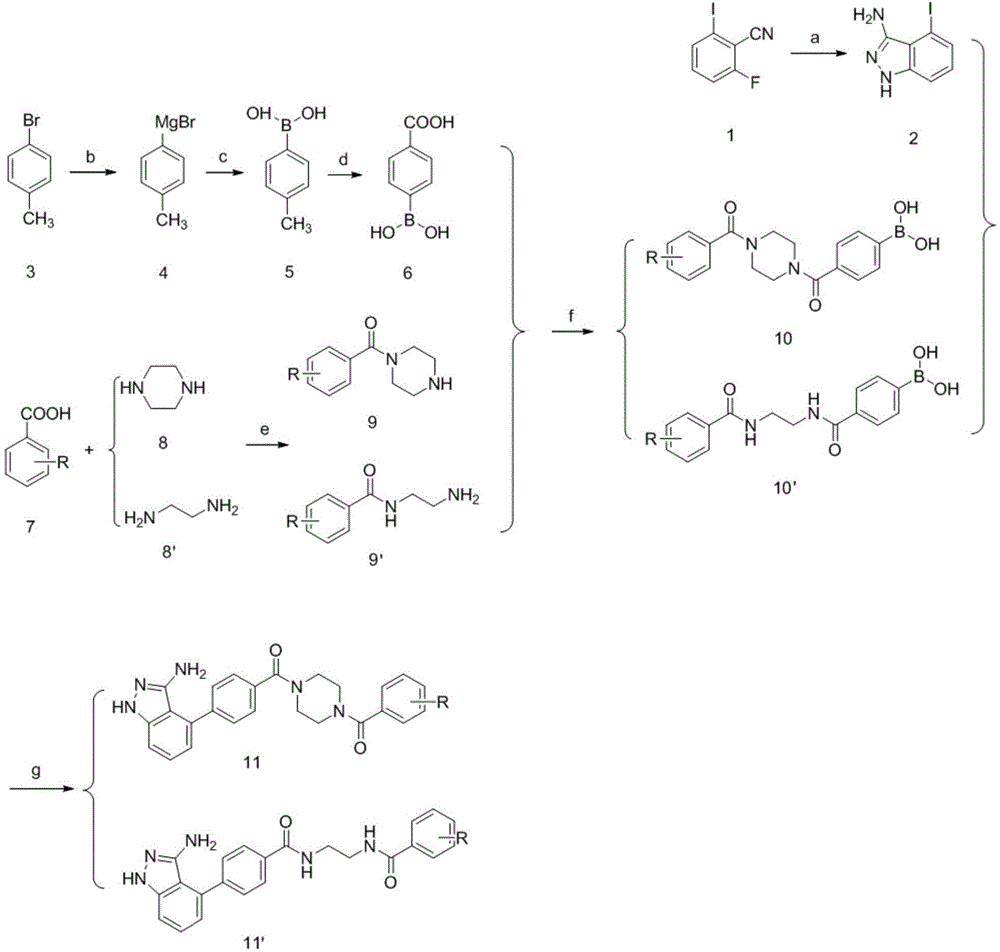
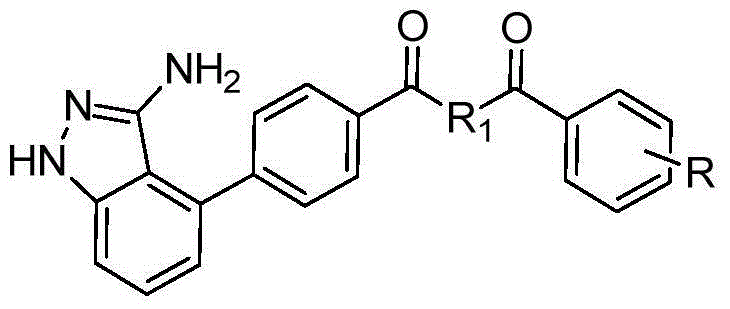
[0040] In the structural formula of this compound, R 1 Is piperazinyl, R is trifluoromethyl, in the meta position, prepared by the following steps (see figure 1 ):
[0041] 1) Hydrazine hydrate and 2-fluoro-6-iodobenzonitrile (compound 1) were refluxed in an alkaline environment to prepare 4-iodo-1H-indazol-3-amine (compound 2);
[0042] Weigh 5g (20.2mmol) of 2-fluoro-6-iodobenzonitrile (compound 1), 2.6g (31mmol) of sodium bicarbonate, and 5g (99.9mmol) of hydrazine hydrate and dissolve it in 25ml of absolute ethanol. The reaction was monitored after heating to reflux for 8 h. After the reaction was complete, cool to room temperature, add 50 ml of water, stir at room temperature for 2 h, filter with suction, and dry the filter cake to obtain 4.8 g of crude product of 4-iodo-1H-indazol-3-amine (compound 2), with a yield of 92%;
[0043] 2) Prepare p-carboxyphenylboronic acid (compound 6) from p-bromotoluene (compound 3) through Grignard reaction, esterification, hydrolysis...
Embodiment 2
[0057] In the structural formula of this compound, R 1 Is piperazinyl, R is a disubstituted chlorine atom, in the ortho and para positions, prepared by the following steps (see figure 1 ):
[0058] Steps 1) to 2) are the same as steps 1) to 2) in Example 1, that is, 4-iodo-1H-indazol-3-amine (compound 1) is prepared from 2-fluoro-6-iodobenzonitrile (compound 1) 2), preparing p-carboxyphenylboronic acid (compound 6) from p-bromotoluene (compound 3);
[0059] 3) Preparation of 1-(2,4-dichlorobenzoyl)piperazine (compound 9) through condensation reaction of 2,4-dichlorobenzoic acid (compound 7) and piperazine (compound 8);
[0060] 100 g of concentrated hydrochloric acid (12 mol / L) was placed in a 250 ml round bottom flask, and 40 g of anhydrous piperazine (compound 8) was slowly added in an ice bath. After the addition, remove the ice bath, react at room temperature overnight, filter with suction, and dry the filter cake in an oven. The obtained white solid is piperazine dihyd...
Embodiment 3
[0070] In the structural formula of this compound, R 1 Is ethylenediamine, R is a methyl group, in the ortho position, prepared by the following steps (see figure 1 ):
[0071] Steps 1) to 2) are the same as steps 1) to 2) in Example 1, that is, 4-iodo-1H-indazol-3-amine (compound 1) is prepared from 2-fluoro-6-iodobenzonitrile (compound 1) 2), preparing p-carboxyphenylboronic acid (compound 6) from p-bromotoluene (compound 3);
[0072] 3) Preparation of N-(2-aminoethyl)-2-methylbenzamide (compound 9') through condensation reaction between o-toluic acid (compound 7) and ethylenediamine (compound 8');
[0073]60g of concentrated hydrochloric acid (12mol / L) was placed in a 250ml round-bottomed flask, and under ice-bath conditions, 40g of anhydrous ethylenediamine (compound 8') was slowly added. After the addition, remove the ice bath, react at room temperature overnight, filter with suction, and dry the filter cake in an oven. The obtained white solid is ethylenediamine dihyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com