Hybridoma cell strain and secretion monoclonal antibody and application thereof
A technology of hybridoma cells and monoclonal antibodies, applied in the direction of antibodies, analytical materials, antiviral agents, etc., can solve the problems of high cost, re-emergence of piglets, single preventive effect of vaccines, etc., and achieve good neutralizing activity and high binding strength Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
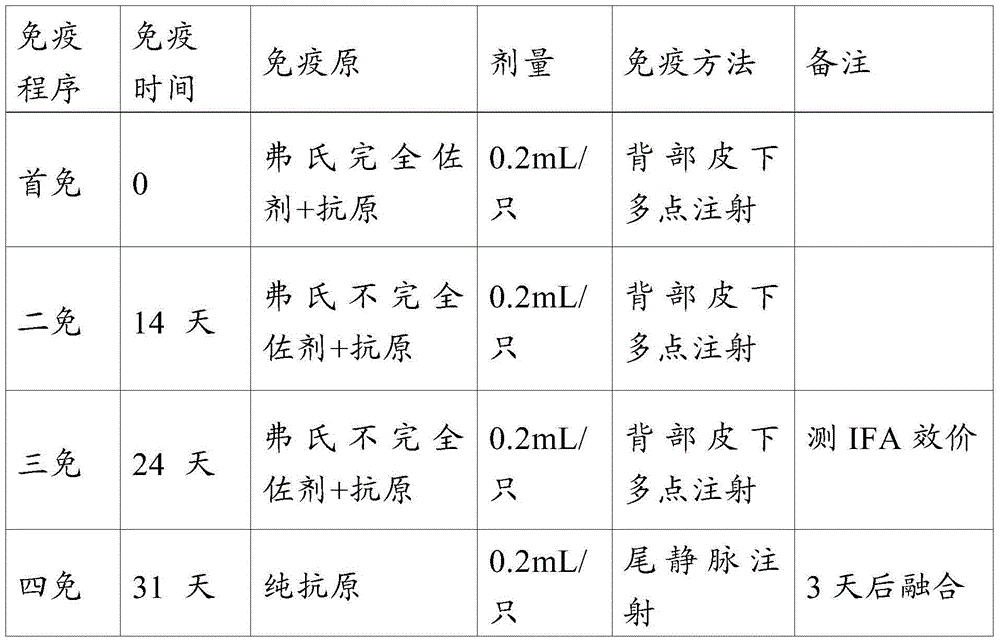
[0056] The acquisition of embodiment 1 hybridoma cell strain
[0057] 1.1 Propagation and purification of PEDV virus
[0058] 1.1.1 Antigen preparation
[0059] Wash the Vero cells that have grown into a good monolayer (the Vero cells are from Shanghai Institute of Biochemistry) for 2-3 times with PBS, and inoculate the PEDV HN1301 strain at a dose of MOI=0.001. Incubate at 37°C for 60 minutes, mix gently every 20 minutes during this period, discard the liquid in the bottle after adsorption, add 5mL of fresh maintenance solution, and place at 37°C CO 2 In the incubator, the virus liquid was harvested when the cytopathic rate reached 90%, and frozen at -20°C for later use.
[0060] 1.1.2 Antigen purification
[0061] Take 200mL of virus liquid and add 20mL TritonX-100, shake at room temperature for 10min, centrifuge at 5000rpm in a high-speed refrigerated centrifuge for 30min, take the centrifuged supernatant in an ultra-speed refrigerated centrifuge at 40000rpm, centrifuge ...
Embodiment 2
[0110] Example 2 Preparation and Identification of Monoclonal Antibody
[0111] 2.1 Preparation of monoclonal antibody ascites First, sensitize BALB / C female mice with sterilized liquid paraffin, inject 0.5 mL / peritoneally, and inject 0.5 mL (about 2.0×10 6 cells / mL) of hybridoma cells. Observation, ascites was taken when the abdomen of the mouse was obviously enlarged. Put the ascitic fluid at 37°C, and then overnight at 4°C after 1 hour. The supernatant was collected by centrifugation the next day, aliquoted, and stored at -70°C.
[0112] 2.2 Identification of monoclonal antibody Ig type
[0113] The Ig type of monoclonal antibody was identified by using the monoclonal antibody typing reagent of Sigma Company according to the instructions. The heavy chain of 8A3A10 monoclonal antibody was IgG2a subtype, and the light chain was Kappa chain.
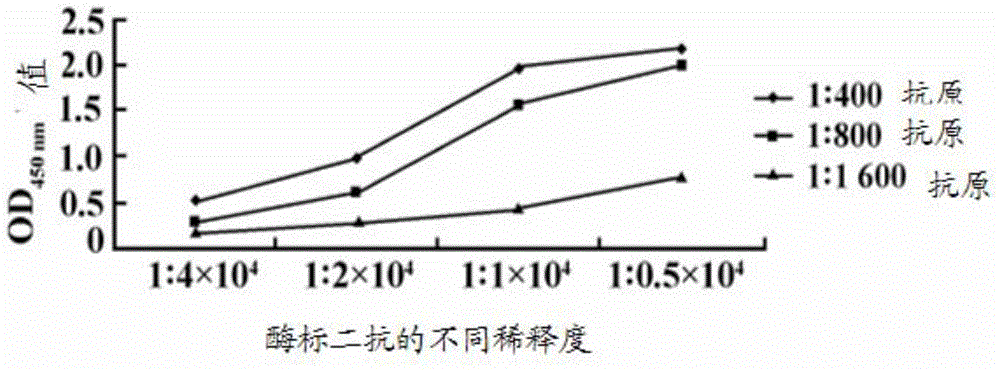
[0114] 2.3 Determination of monoclonal antibody titer
[0115] Using the IFA method in Example 1.4.2, the IFA titer of the prepare...
Embodiment 3
[0134] Example 3 Monoclonal Antibody Prevention, Effect Evaluation of Treatment Virus Infection
[0135] 3.1 Evaluation of the effect of monoclonal antibody in preventing viral infection
[0136] Screen PEDV, RV, TGEV antigen, 15 2-3 day-old piglets of double-negative age of antibody, be divided into 3 groups at random, the monoclonal antibody 2mL that the first group and the second group are prepared in oral embodiment 2 respectively, the third group orally 2 mL of PBS buffer, 24 hours after oral administration, the first group and the third group were orally administered 2 mL of PEDV epidemic strain HN1301 small intestinal virus (containing 1000 minimum incidence doses), and the second group were orally administered CV777 small enterotoxin (containing 1000 minimum incidence doses), The clinical symptoms of piglets were observed daily for 14 consecutive days, and the effect of the monoclonal antibody on preventing diarrhea virus infection was evaluated by the clinical morbidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com