Method for production of ethanol and co-production of methanol
A technology of methanol and ethanol, applied in the field of catalytic chemistry, to achieve the effect of improving product flexibility and high hydrogenation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
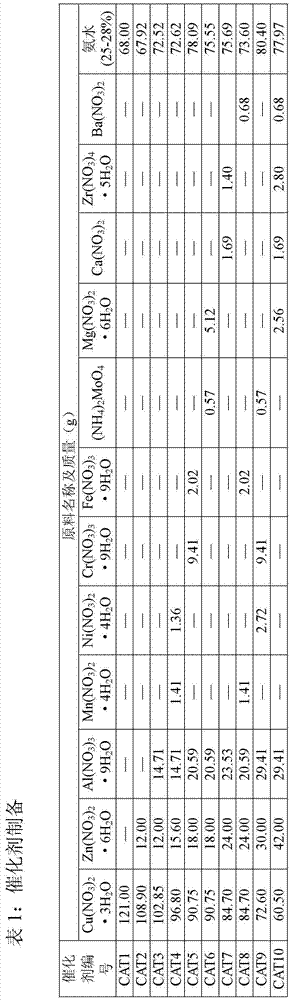
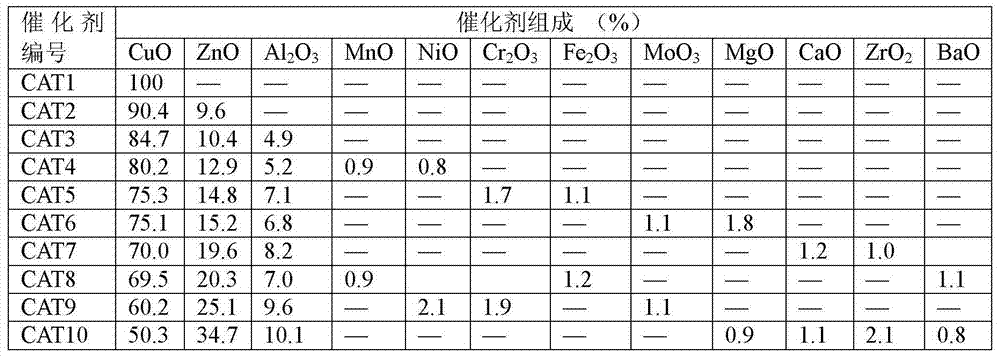
[0029]Embodiment 1: catalyst preparation
[0030] 1) Preparation of 100% CuO catalyst
[0031] 121g Cu(NO 3 ) 2 ·3H 2 O was dissolved in 2000ml of deionized water, and 68.0g of concentrated ammonia (25-28%) was diluted with 1500ml of deionized water. Stir the ammonia solution vigorously at room temperature, then slowly add the metal nitrate solution into the ammonia solution for about 60 minutes. Adjust the pH value of the precipitate to 10.0 with ammonia solution, continue to stir for 200 min, and age for 36 h. The precipitate was washed with deionized water until neutral, and centrifuged. The obtained precipitate was dried in an oven at 120°C for 24 hours, and the dried sample was placed in a muffle furnace, heated to 400°C at a heating rate of 1°C / min, and calcined for 5 hours to obtain a calcined sample. This catalyst is designated CAT1.
[0032] 2) 85%CuO / 10%ZnO / 5%Al 2 o 3 Catalyst preparation
[0033] 102.85g Cu(NO 3 ) 2 ·3H 2 O, 12.00g Zn(NO 3 ) 2 ·6H 2 ...
Embodiment 2
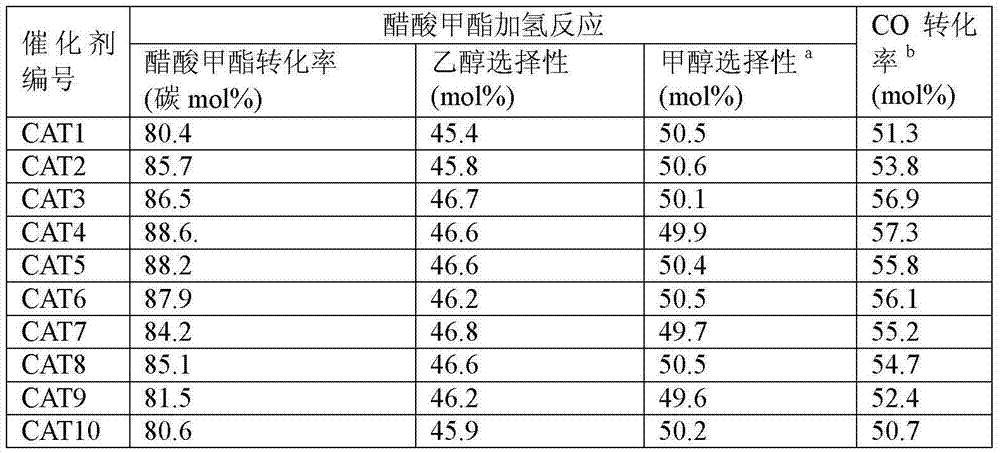
[0040] Example 2: Catalyst Evaluation
[0041] The reaction volume space velocity in the present invention is defined as the volume flow of the reaction raw materials (under standard conditions) entering the reaction system per hour divided by the mass of the catalyst. In GHSV, the unit is mlg -1 h -1 .
[0042] 10g of the above-mentioned catalyst of 20-40 mesh is loaded into the constant temperature zone of the fixed-bed reactor. Before the reaction, the catalyst was reduced online, the reduction temperature was 260°C, the pressure was 0.1MPa, and the reducing gas was 5% H 2 +95%N 2 , Reduction time 24h. After the reduction, adjust the temperature controller to reduce the reaction temperature to 230°C, and use N 2 Purge the pipeline and the residual H in the reactor 2 , and then switch the gas to a certain composition of synthesis gas and pressurize it, adjust the mass flow meter to the specified flow rate (standard condition), set the acetate high-pressure feed pump t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com