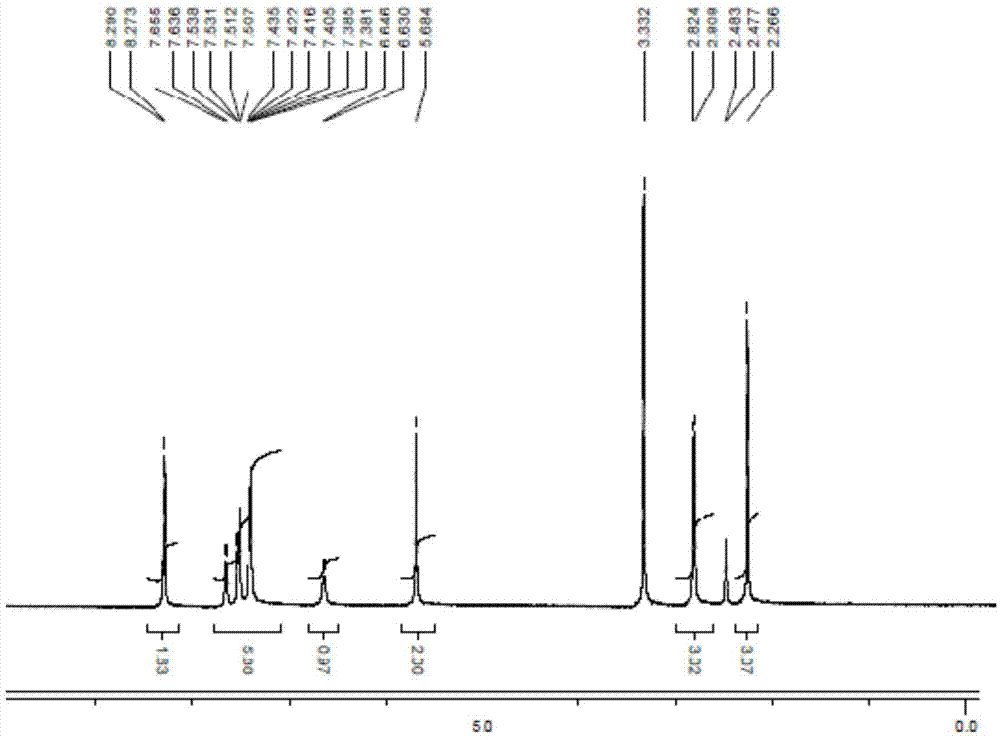
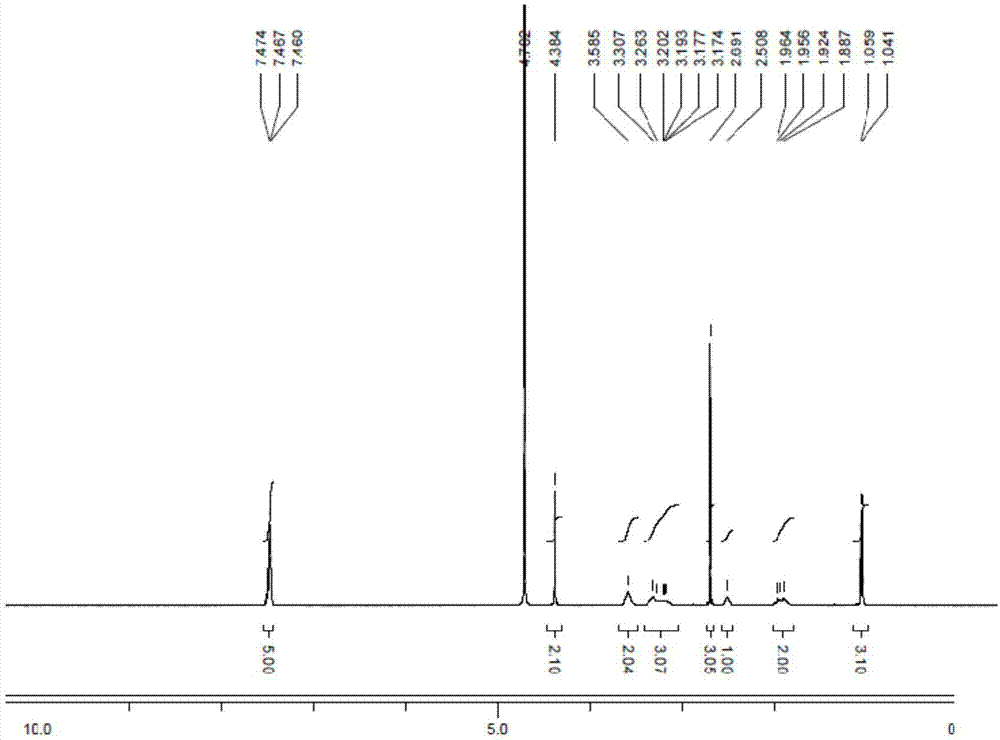
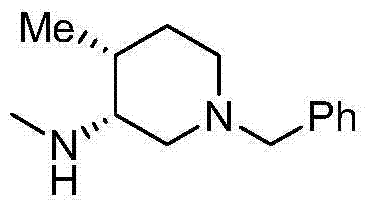
Method for synthesizing cis-1-benzyl-3-methylamino-4-methyl-piperidine
A technology of methylamino and aminopyridine, which is applied in the field of synthesizing cis-1-benzyl-3-methylamino-4-methyl-piperidine, can solve the problem of high synthesis cost, achieve high cis-trans selectivity, improve Synthesis efficiency, effect of reducing synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
preparation Embodiment 1
[0031] Preparation Example 1: Preparation of Compound 6 and Synthetic Intermediates
[0032] Potassium tert-butoxide (20.7 g, 0.18 mol) was added to a 250 mL reaction flask, 2-methyltetrahydrofuran (100 mL) was added, and stirred. In an ice bath <25°C, add dimethyl carbonate (10g, 0.11mol) dropwise, add 4-amino 3-methylpyridine 10 (10g, 0.093mol), raise the temperature to 25°C for 4 hours, and monitor the raw materials by HPLC ≤1%. The reaction solution was slowly added to water (50 mL), stirred at <20°C for 10 minutes, separated, the aqueous phase was extracted once with 2-methyltetrahydrofuran (20 mL), and the organic phases were combined. The organic phase was washed once with 20% brine (10 mL), separated, and the organic phase was taken. The organic phase was concentrated to 20 mL, n-heptane (80 mL) was added, and stirred for 1.5 hours. Filter, and dry the filter cake at 45°C under normal pressure for 12 hours, LOD<0.5%. Product 14 (14.2 g, yield 92%) was obtained.
...
preparation Embodiment 2
[0036] Preparation Example 2: Preparation of Compound 16
[0037] Add 3-methylamino4-picoline 15 (30.4 g, 0.249 mol) into the reaction flask, add acetone (300 mL), and stir to dissolve. Measure the water content, if >5000ppm, then azeotropic water removal. Benzyl bromide (44.7g, 0.261mol) was dropped into the reaction flask at room temperature, a large amount of white solids precipitated, continued to stir for 1 hour, heated to 60°C and refluxed for 2 hours, HPLC monitored that the liquid contained less than 1% of raw materials, cooled to At room temperature, filter, and wash the filter cake with acetone (30 mL) three times. The filter cake was collected and dried under normal pressure at 45° C. for 16 hours to obtain a near-white solid 16 (69.0 g, yield 94%).
preparation Embodiment 3
[0038] Preparation Example 3: Preparation of Compound 6
[0039] Add ethanol (70mL) to the three-necked reaction flask, add compound 16 (10g, 0.034mol), stir, heat to an internal temperature of about 35°C to dissolve, and slowly add NaBH in batches 4 (3.9g, 0.11mol), there will be gas released during the feeding process, the temperature of the system will gradually increase, after the feeding is completed, heat to an internal temperature of 65°C and keep it warm for 2 hours, control the sampling, raw materials <2%, concentrate ethanol to a small volume. Add isopropyl acetate (50 mL) and tap water (50 mL) to the concentrated residue, stir for 2 h, let stand to separate the layers, and separate the water layer. Water (50 mL) was added to the organic phase again, stirred for 1 h, the layers were separated, and the water layer was separated. 10% brine (10 mL) was added to the organic phase to wash for 1 h, the layers were separated after standing, and the water layer was separate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com