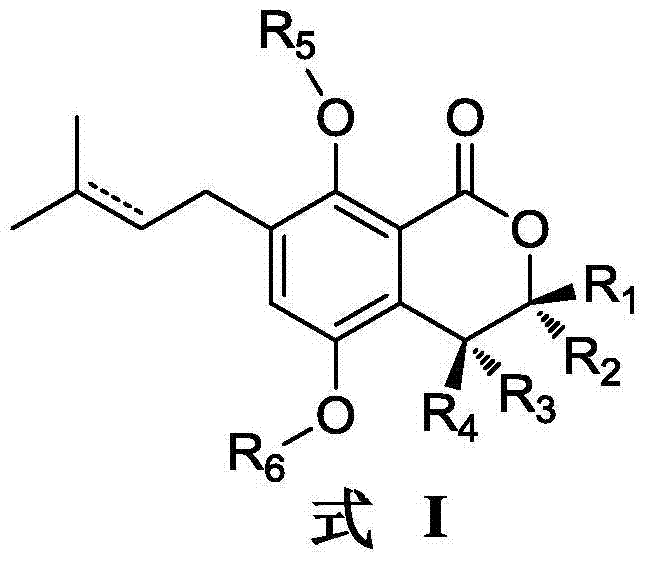
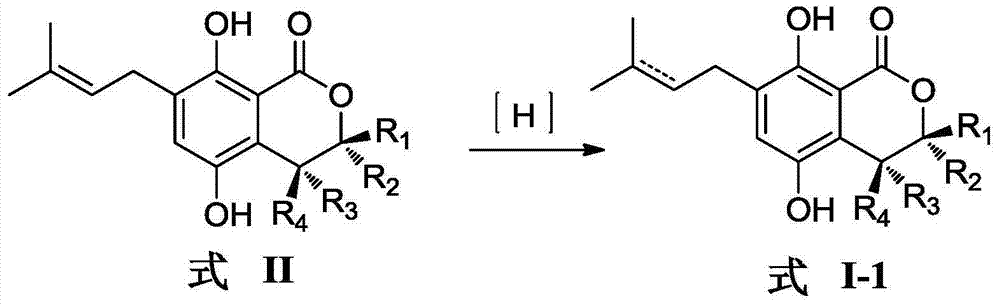
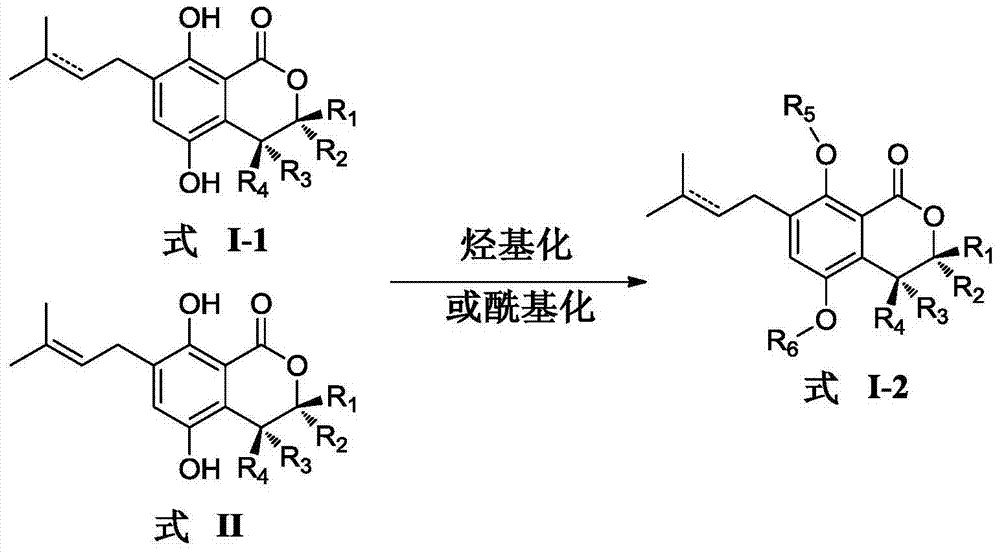
Gorgonian-originated fungus secondary metabolite derivatives and application of same as antiseptics
The technology of an antibacterial agent and a compound is applied in the field of derivatives of isocoumarin compounds of secondary metabolites of gorgonian fungus and its preparation, and can solve the problems of difficult clinical treatment, unreasonable clinical misuse and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032]Weigh 20 mg of compound 1 and dissolve it in 5 mL of THF, add 5 mg of NaH, stir at room temperature for half an hour, add 20 μL of MeI, react at 60 ° C for 12 h, TLC detects that the reaction raw materials almost completely disappear, add saturated ammonium chloride to terminate the reaction, and use Extracted twice with ethyl acetate, dried the organic layer with anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography (eluent: EtOAc / petroleum ether=20:1~10:1) to obtain compound 2 (9.5 mg), Yield 45.9%, ESI-MS m / z: 387.2[M+Na] + , compound 3 (8.5mg) yield 39.5%, ESI-MS m / z: 401.2[M+Na] + , and the purity detected by HPLC is above 97.3%.
Embodiment 2
[0034]
[0035] Weigh 10mg of compound 1 and dissolve in 5mL CH 2 Cl 2 Add 10 μL of pyridine and 10 μL of acetic anhydride, react at room temperature for 2 hours, TLC detects that the reaction raw materials almost completely disappear, after concentration, go through silica gel column chromatography (eluent: EtOAc / petroleum ether=15:1~10:1) , to obtain compound 4 (10.1mg), yield 90.5%, compound HPLC detection purity 98.3%, ESI-MSm / z: 415.2[M+Na] + .
Embodiment 3
[0037]
[0038] Weigh 20 mg of compound 1 and dissolve in 5 mL of THF, add 20 μL of pyridine, catalytic amount of DMAP, 30 μL of ClCH 2 COCl, after reacting at 60°C for 8 hours, TLC detected that the reaction raw materials almost completely disappeared, extracted twice with ethyl acetate, dried the organic layer with anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography (eluent: EtOAc / petroleum ether) =20:1~10:1), compound 6 (14.5mg) was obtained, the yield was 50.8%, ESI-MS m / z:525.1[M+Na] + , compound 5 (11.0mg) yield 45.2%, ESI-MS m / z: 449.1[M+Na] + , and the purity detected by HPLC is above 96.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com