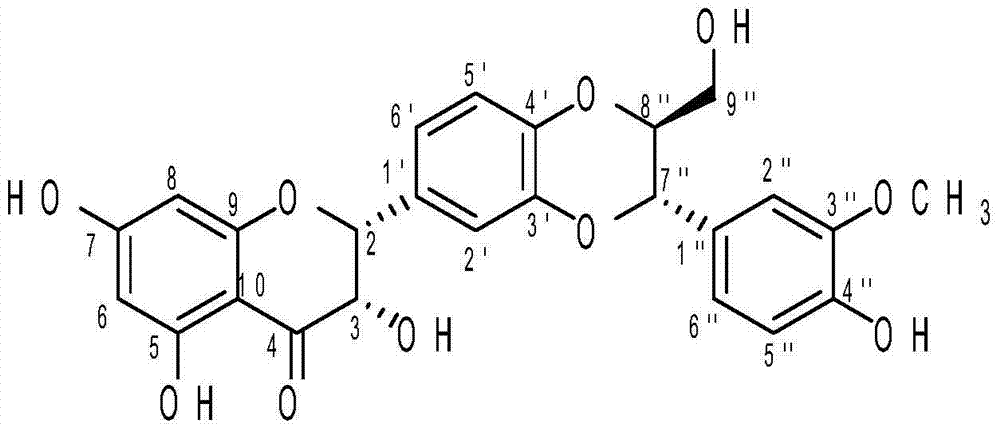
Preparation method of 2,3-cis-silybin B
A silibinin and semi-preparative chromatographic technology, applied in the field of preparation of 2,3-cis-silibinin B, can solve the problems of low content, inability to prepare high-purity compounds, and no separation to obtain high-purity compounds. Achieving shortened process, reduced sample and solvent consumption, fast and efficient method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
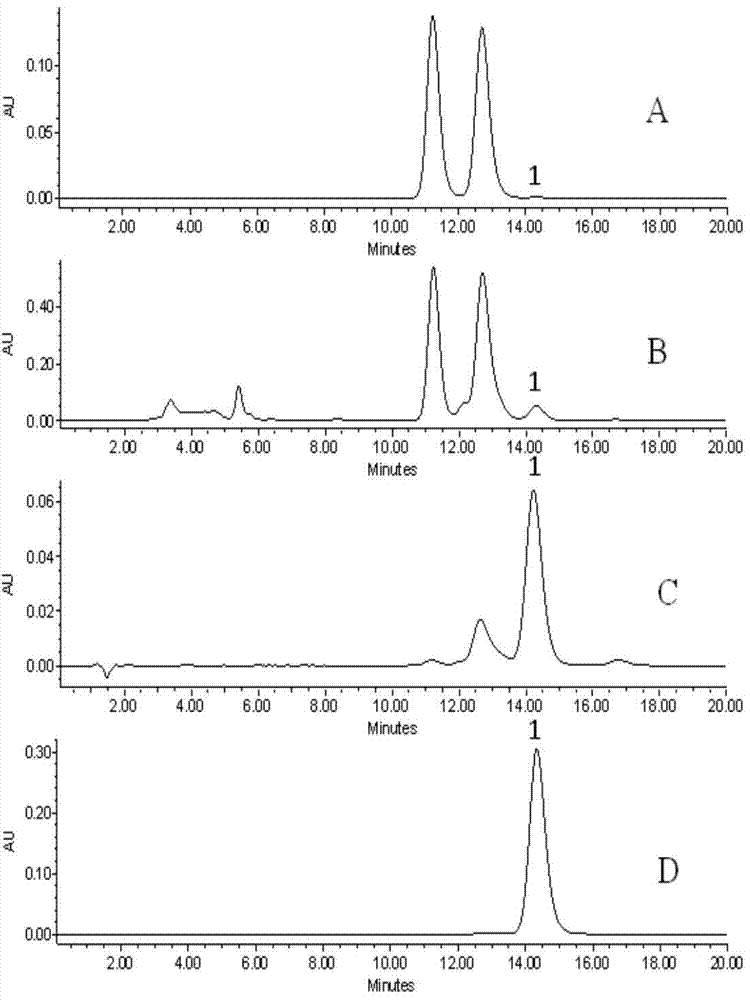
[0028] 10g silybin mixture (for analysis see figure 1 A) Mix with 5g Na2CO3 and dissolve in 40% methanol, heat to reflux for 12 hours, cool, neutralize the reaction solution to pH7 with HCl, filter to obtain 2,3-cis-silibinin B conversion with a purity of 3% (Analysis see figure 1 B). The transformed product was separated by an industrial chromatographic column (190×80mm, 15um) and eluted with 55% methanol. The fractions containing the target compound were collected and concentrated under reduced pressure to obtain 2,3-cis-silibinin B-rich with a purity of 70%. Collection (analysis see figure 1 C), the enrichment was separated by a semi-preparative chromatographic column (250×20mm, 5um), eluted with 60% methanol, and the fraction containing the target compound was collected and concentrated under reduced pressure to obtain 2,3-cis with a purity of 98.4%. - silybin B product (analysis see figure 1 D).
Embodiment 2
[0030] 10g silybin mixture and 100g NaHCO3 were mixed and dissolved in 100% methanol, heated to reflux for 16 hours, cooled, neutralized with HCl to pH7, filtered to obtain 2,3-cis-silymarin with a purity of 3% Bin B transformants. The transformed product was separated by an industrial chromatographic column (190×80mm, 15um) and eluted with 55% methanol. The fractions containing the target compound were collected and concentrated under reduced pressure to obtain 2,3-cis-silibinin B-rich with a purity of 30%. The enriched material was separated by a semi-preparative chromatographic column (250×20mm, 10um) and eluted with 60% methanol. The fraction containing the target compound was collected and concentrated under reduced pressure to obtain 2,3-cis with a purity of 95.5%. - a silybin B product.
Embodiment 3
[0032] Mix 0.1g of silybin B monomer compound with 0.5ml of ammonia water and dissolve in 40% ethanol, heat to reflux for 24 hours, cool, neutralize the reaction solution with HCl to pH7, and filter to obtain 2,3- Cis-silibinin B transformant. The transformed product was separated by an industrial chromatographic column (240×100mm, 45um) and eluted with 55% methanol. The fractions containing the target compound were collected and concentrated under reduced pressure to obtain 2,3-cis-silibinin B-rich with a purity of 50%. The enriched material was separated by a semi-preparative chromatographic column (250×10mm, 5um) and eluted with 55% methanol. The fraction containing the target compound was collected and concentrated under reduced pressure to obtain 2,3-cis with a purity of 97.3%. - a silybin B product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com