Improved method for preparing d-biotin from bisbenzyl biotin by debenzylation
A technology of bisbenzyl biotin and biotin, applied in the field of chemical synthesis of d-biotin, can solve the problems of poor environmental protection, poor product quality and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
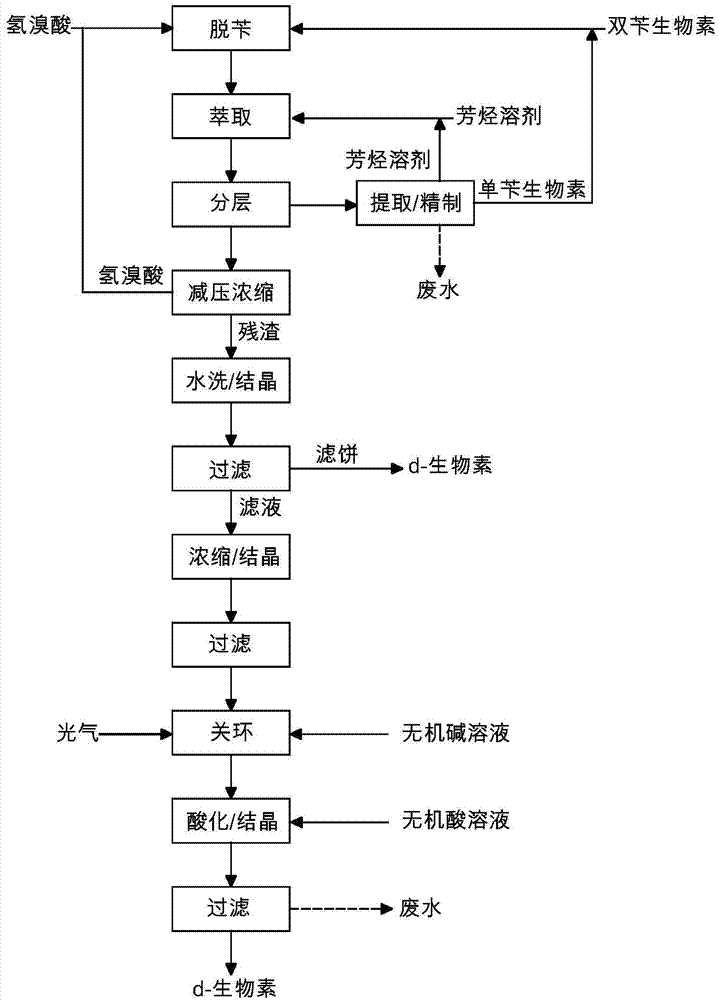
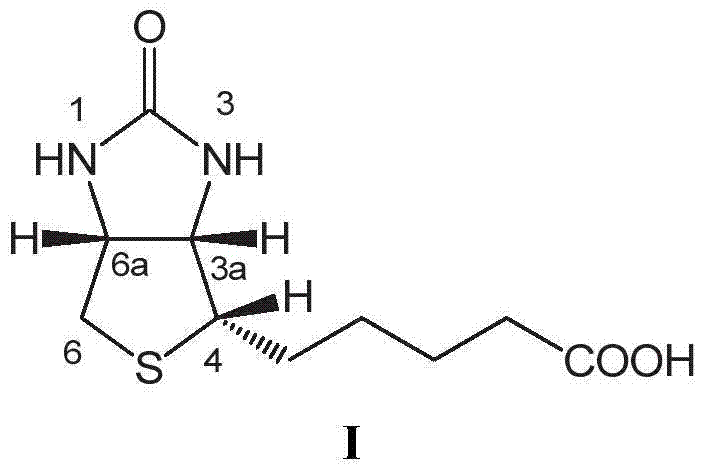
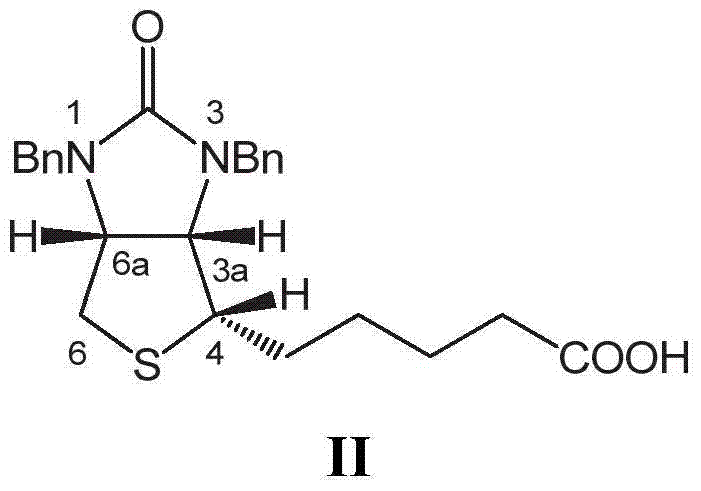
[0063]20g of dibenzyl biotin shown in formula II is placed in a three-necked flask, adding 200g (about 135ml) concentration is 48% hydrobromic acid, warming up to reflux, and separating bromobenzyl, keep reflux until TLC detection (developing Agent: ethyl acetate: ethanol=1:1) When showing no raw material point, the reaction ends. Cool down to room temperature, add 20ml×3 toluene for extraction three times, separate the aqueous phase, and combine the organic phases. The organic phase was distilled, and about 45 ml of toluene was distilled off. The distillation residue was cooled to room temperature, allowed to stand for 10 hours, and filtered to obtain 2.8 g of monobenzyl biotin of formula IVa and / or formula IVb. About 7ml of the filtered organic phase crystallization mother liquor is to be applied to the next batch.
[0064] The aqueous phase was distilled to dryness under reduced pressure to recover hydrobromic acid. Add 50ml of pure water to the distillation residue, sti...
Embodiment 2
[0068] With 20g of dibenzyl biotin shown in formula II and 2.8g of monobenzyl biotin recovered in Example 1, add 250g (about 175ml) of hydrobromic acid with a concentration of 40%, heat up to reflux, and separate bromine Benzyl, keep refluxing until TLC detection (developing solvent: ethyl acetate: ethanol = 1:1) shows no raw material point, the reaction is over. Cool down to room temperature, add 60ml of cumene to extract once, and separate the aqueous phase and the organic phase. The organic phase was combined with the organic phase crystallization mother liquor in Example 1, and evaporated under reduced pressure to distill out about 52 ml of the mixed solvent. The distillation residue was cooled to -5°C, allowed to stand for 2 hours, filtered and recrystallized with pure water to obtain 4.4 g of monobenzyl biotin of formula IVa and / or formula IVb. About 8ml of the filtered crystallization mother liquor is to be applied to the next batch.
[0069] The aqueous phase was con...
Embodiment 3
[0073] With 20g of dibenzyl biotin shown in formula II and 4.4g of monobenzyl biotin recovered in Example 2, add 120g (about 80ml) of hydrobromic acid with a concentration of 55%, heat up to reflux, and separate the bromine Benzyl, keep refluxing until TLC detection (developing solvent: ethyl acetate: ethanol = 1:1) shows no raw material point, the reaction is over. Cool down to room temperature, add 15ml×5xylene for extraction five times, separate the aqueous phase, and combine the organic phases. The organic phase is combined with the organic phase crystallization mother liquor in Example 2, back-extracted three times with 15% NaOH solution 10ml × 3, the organic phase is separated, the aqueous phase is combined, the pH value is adjusted to 2 with 15% hydrobromic acid, and left to stand for 8 hours , and filtered to obtain 6.5 g of monobenzyl biotin.
[0074] The separated aqueous phase was concentrated to dryness under reduced pressure to recover hydrobromic acid. Add 20ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com