Preparation method of CuS/g-C3N4 nano-spheroidized compound catalyst
A technology of g-c3n4 and nanospheres, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as photochemical corrosion and poor stability, and achieve easy operation, improved stability, and guaranteed The effect of adsorption and catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) g-C 3 N 4 preparation of
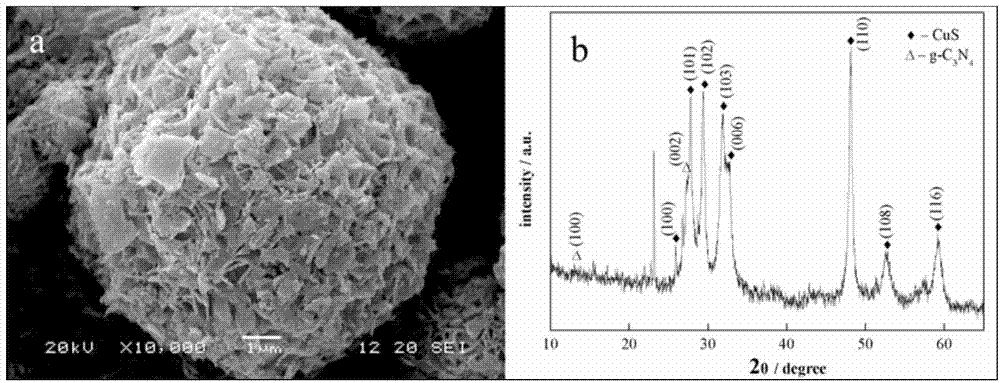
[0019] Weigh 3.0g of melamine into a 50mL ceramic crucible, put it into a muffle furnace, and raise the temperature from room temperature to 550°C in the air at a rate of 2°C / min. After constant temperature for 4 hours, cool naturally to room temperature to obtain yellow g-C 3 N 4 product.
[0020] (2) CuS / g-C 3 N 4 Preparation of nanosphere flowers
[0021] Weigh copper acetate, acetylacetone and sulfur chloride respectively, g-C 3 N 4 In a beaker, add absolute ethanol and ultrasonically stir for 10 min. Each raw material mass ratio is copper acetate: acetylacetone: sulfur chloride: g-C 3 N 4 = 1:1:3:0.44. Add acetylacetone-ethanol solution and sulfur chloride-ethanol solution to copper acetate-ethanol solution respectively, then add g-C 3 N 4 - After adding the ethanol solution to the above mixed solution, sonicate for 20 minutes. The mixed solution was transferred to a polytetrafluoroethylene reactor and heated in an oven ...
Embodiment 2
[0023] CuS / g-C 3 N 4 Preparation of nanosphere flowers
[0024] Weigh copper acetate, acetylacetone and sulfur chloride respectively, g-C 3 N 4 (Preparation is the same as in Example 1) In a beaker, add absolute ethanol and ultrasonically stir for 10 min. Each raw material mass ratio is copper acetate: acetylacetone: sulfur chloride: g-C 3 N 4 = 1:1:3:0.44. Add acetylacetone-ethanol solution and sulfur chloride-ethanol solution to copper acetate-ethanol solution respectively, then add g-C 3 N 4 - After adding the ethanol solution to the above mixed solution, sonicate for 20 minutes. The mixed solution was transferred to a polytetrafluoroethylene reactor and heated in an oven at 160°C for 24h. Wash the precipitate with alcohol three times, and dry at 60°C to obtain CuS / g-C 3 N 4 Nanosphere flower composite catalyst.
Embodiment 3
[0026] CuS / g-C 3 N 4 Preparation of nanosphere flowers
[0027] Weigh copper acetate, acetylacetone and sulfur chloride respectively, g-C 3 N 4 (Preparation is the same as in Example 1) In a beaker, add absolute ethanol and ultrasonically stir for 10 min. Each raw material mass ratio is copper acetate: acetylacetone: sulfur chloride: g-C 3 N 4 =1:1:3:0.9 (marked as A), or copper acetate: acetylacetone: sulfur chloride: g-C 3 N 4 =1:1:3:0.67 (marked as B), or copper acetate: acetylacetone: sulfur chloride: g-C 3 N 4 =1:1:3:0.11 (denoted as C). Add acetylacetone-ethanol solution and sulfur chloride-ethanol solution to copper acetate-ethanol solution respectively, then add g-C 3 N 4 - Add the ethanol solution to the above mixed solution respectively, and sonicate for 20 minutes. The mixed solution was transferred to a polytetrafluoroethylene reactor and heated in an oven at 160 °C for 2 h. Wash the precipitate with alcohol three times, and dry at 60°C to obtain CuS / g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com