Non-pathogenic F18 E. coli strain and use thereof
An Escherichia coli, non-pathogenic technology, applied in the direction of bacteria, blood diseases, antibacterial drugs, etc., can solve the problems of low reliability and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a) Non-pathogenic F18 E. coli strain
[0032] For Escherichia coli (EcL), Dr. J.M. Fairbrother isolated large intestine from pig feces in 1996 at the OIE reference laboratory (Faculté de medicine vétérinaire (FMV), Université de Montréal (UdeM), Saint-Hyacin, Quebec, Canada) Bacillus strain, said Escherichia coli strain was deposited in the International Depository Authority of Canada (IDAC) on June 20, 2013 and has an accession number of 200613-01.
[0033] Identification of the strains was performed using the API system. The API 20E code for Master Seed is 5004552. The serotype of the strain is shown as O141:K94:H-serotype.
[0034] The virulence type of the strains was determined by colony hybridization and / or polymerase chain reaction (PCR). Virulence type results show that the strain is positive for F18 and AIDA, but negative for the following toxins: STa, STb, LT, EAST1, Stx1, Stx2, CNF, F4, F5, F6, F17, F41, P, AFA, Eae , Paa, Tsh, and aerogenin genes. There...
Embodiment 2
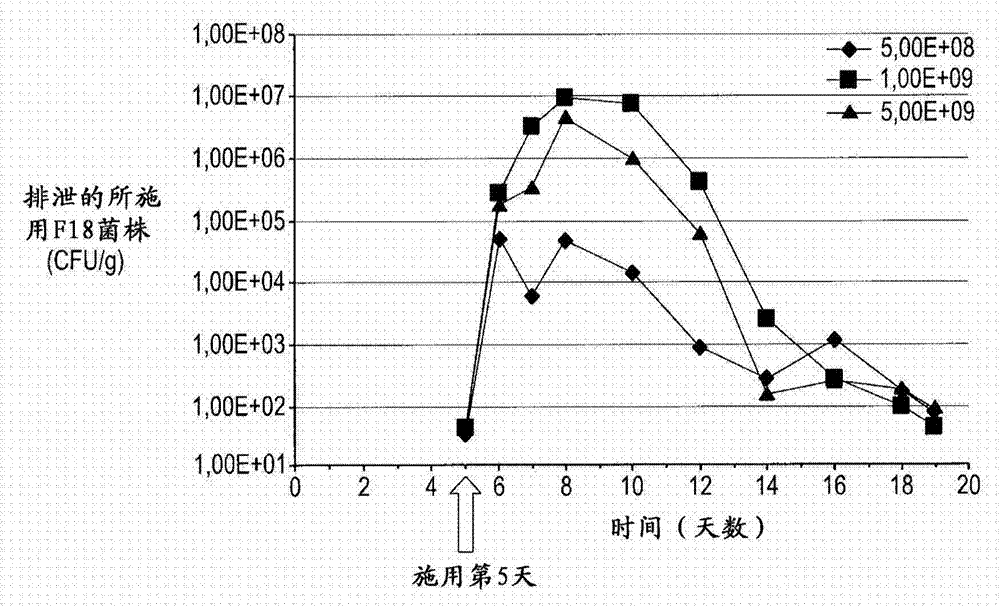
[0039] In one broad aspect, the purpose of the study was to evaluate the effect of different oral doses of the non-pathogenic F18 strains described herein on their fecal excretion and anti-F18 systemic humoral response.
[0040] Table 1 below generally outlines the research schedule:
[0041]
[0042]
[0043] I-Study Design
[0044] a) animals
[0045] Blood samples from 50 lactating piglets were collected for F18 receptor status analysis, and the piglets' ears were marked. Selected weaner pigs (male and female) aged between 17 and 20 days were purchased from a local farm in Montérégie, Quebec, Canada. Transport animals to an isolation facility. Selected farms were free of recent (less than 6 months) Porcine Reproductive and Respiratory Syndrome (PRRS) and post-weaning disease events. Pigs were healthy pigs and weighed between 4Kg and 6Kg. A total of twenty-one (21) pigs positive for F18 receptor status were used and randomized using ear tag numbers into three (3) ...
Embodiment 3
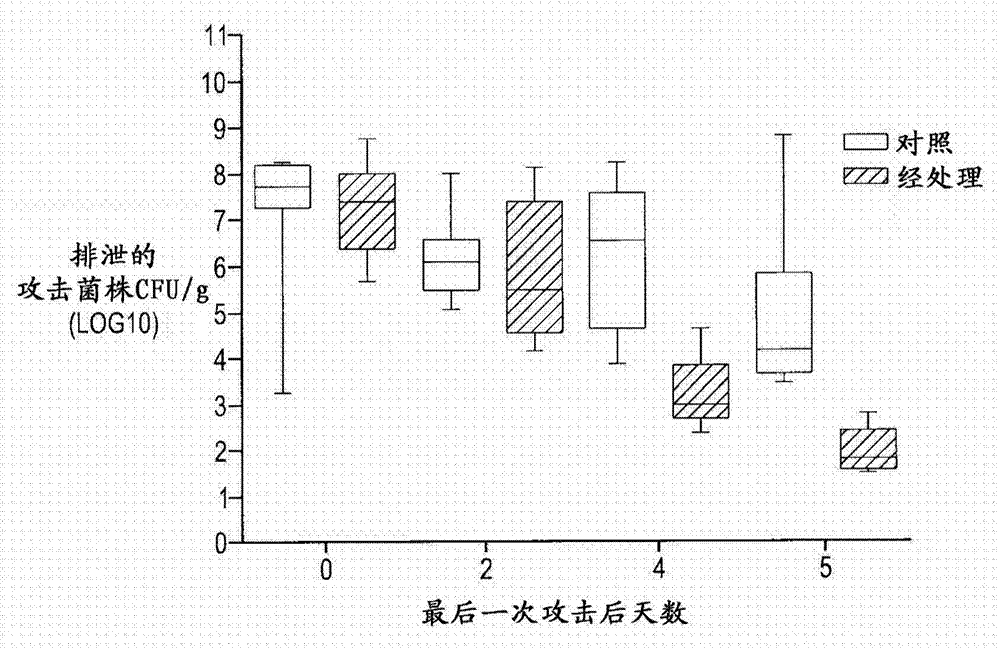
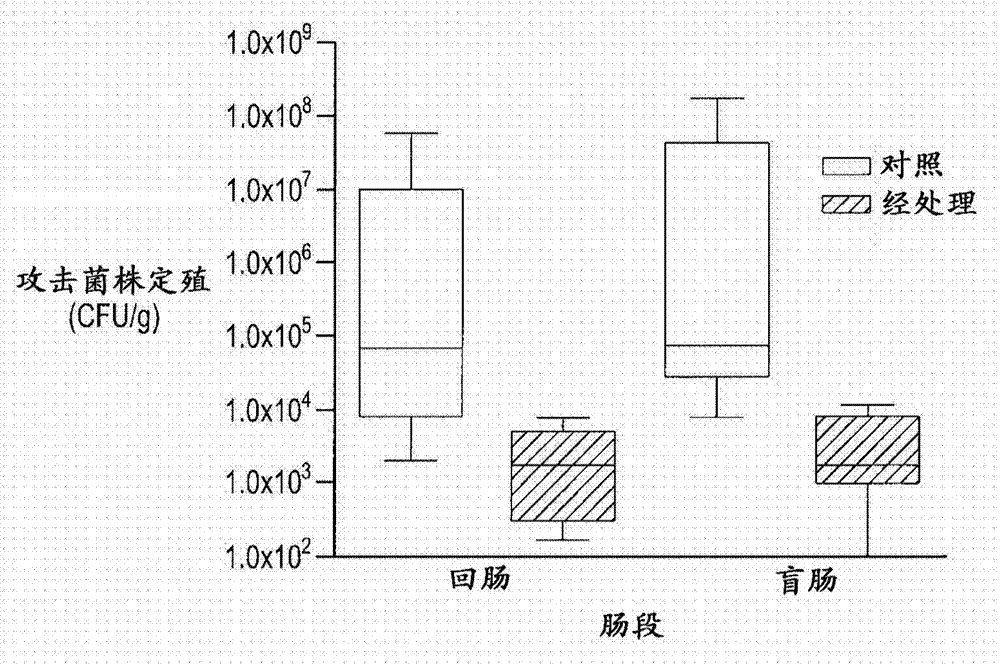
[0102] In one broad aspect, the purpose of the study was to evaluate the efficacy of a composition comprising a combination of the non-pathogenic F4 and F18 E. coli strains described herein.
[0103] Table 4 below generally outlines the research schedule:
[0104] number of days
event
0
Pigs arrive at the experimental unit and are divided into 2 groups
0 to 5
Adaptation period
1, 12, 14, 16, 18 and 19
with stool sampling for PCR
4, 12, 17 and 19
blood sample
5
12, 13 and 14
to attack
12, 14, 16, 18 and 19
Fecal sampling for viable counts of challenge strains
19
Final autopsy and end of experiment
[0105] I-Study Design
[0106] a) animals
[0107] Blood samples from 50 lactating piglets were collected for F18 receptor detection and analysis, and the piglets' ears were marked. Selected weaner pigs (male and female) aged between 17 and 21 days wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com