An alkynol preparing method
A technology of acetylenic alcohol and acetylene, which is applied in the field of chemical intermediate preparation, can solve the problems of difficult stability of single substitutions and low yield, and achieve the effects of increasing yield, increasing safety, and eliminating the existence of air
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
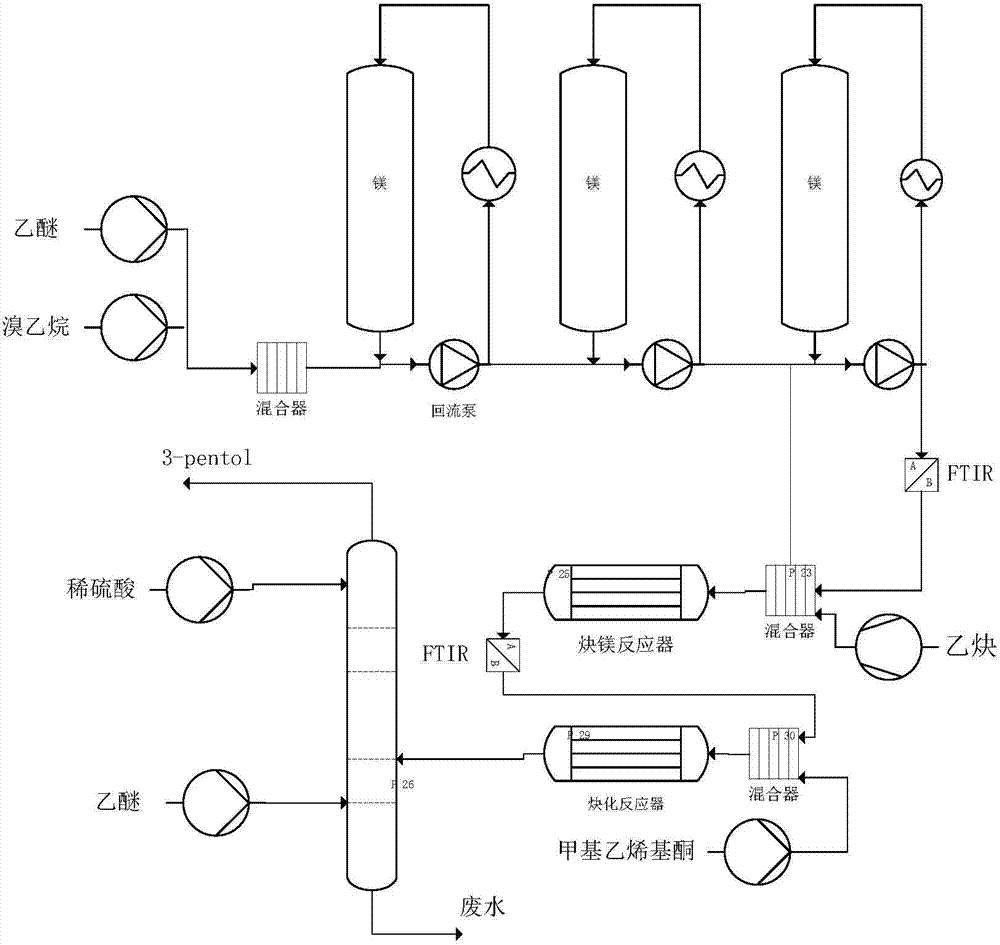
[0057] (1) In a 500ml glass reactor to be pressed with a FTIR (Fourier transform infrared spectrometer) probe and a thermometer, add 4.8g of magnesium flakes, and then add 300ml of diethyl ether containing 21.8g of bromoethane. Stir until the magnesium flakes disappear (you can add a small amount of iodine to initiate). Then lower the temperature to -10°C and slowly pass through acetylene without acetone until it turns white. Close the vent and raise the acetylene pressure to 3.0 bar. React until there is no acetylene gas absorption, and the FTIR curve scan shows that the peak height of the bilateral acetylene disappears, and the unilateral acetylene product is obtained;
[0058] (2) Keep the acetylene pressure at 3.0 bar and the kettle temperature at about 0°C, slowly pump 13 g of methyl vinyl ketone (content 99.5%) into the reaction system of step (1). After that, react for 1 h. Return to normal pressure, keep the reaction solution at about 0°C and slowly drop in 10% dilu...
Embodiment 2
[0060] The operation method is basically the same as in Example 1, except that the methyl vinyl ketone in step (2) in Example 1 is replaced by β-ionone, the reaction temperature is 5°C, and the hydrolysis temperature is 5°C. Ethynyl-β-ionol was obtained with a yield of 96% after post-treatment.
Embodiment 3~7
[0062] The reaction conditions of Examples 3-7 are basically the same as those of Example 1, the substrate and pressure are as shown in Table 1, and the results obtained are listed in Table 1.
[0063] The reaction conditions and the result of embodiment 3~7
[0064] Example
[0065] 7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com