Purifying method of (E)-2-phenyl-3-(3,5-dimethoxy-4-isopropyl benzene) crylic acid
A technology of dimethoxy, cumene, applied in the field of pharmacy, can solve problems such as inability to achieve mass production, large loss of acrylic acid, and difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 One kind ( E )-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid purification method
[0043] A sort of( E )-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid purification method, it comprises two steps:
[0044] The first step is to purify 80% ( E )-2-phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid was subjected to selective esterification reaction, and the purity of 90% ( E )-2-Phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid a; the reaction proceeds according to the following equation:
[0045]
[0046] Follow the steps below in order:
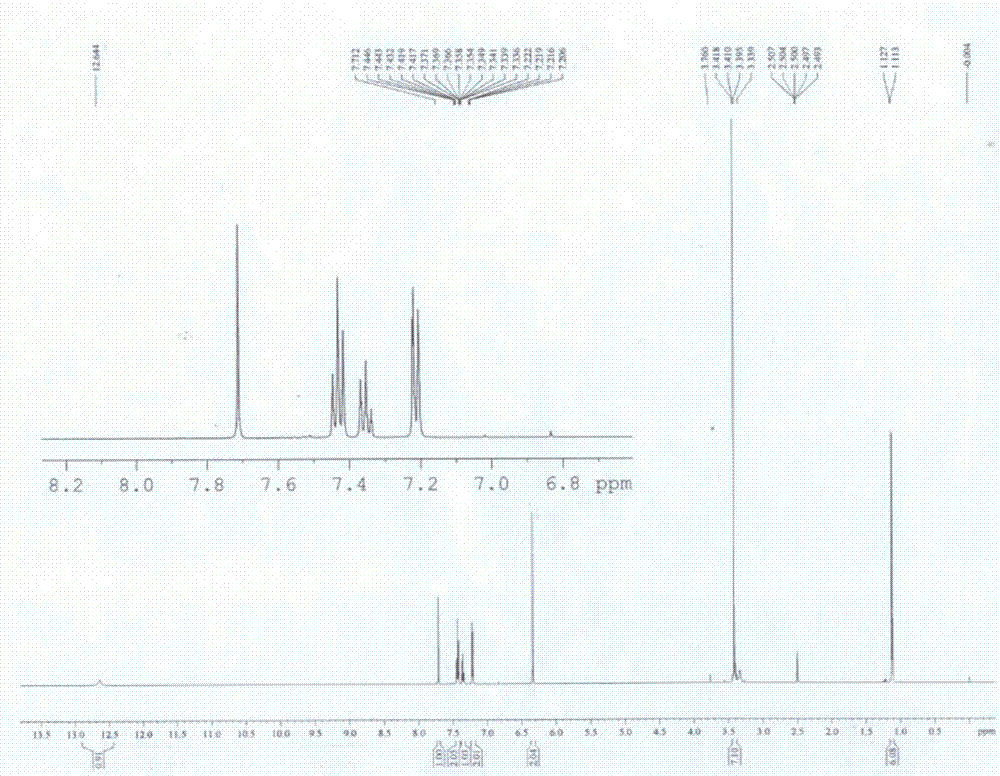
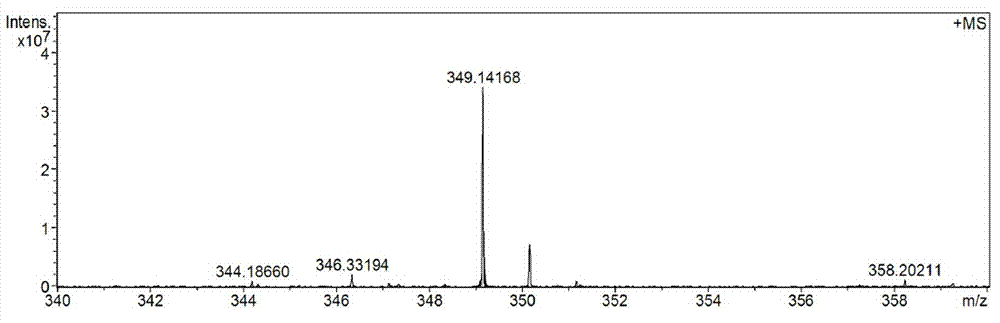
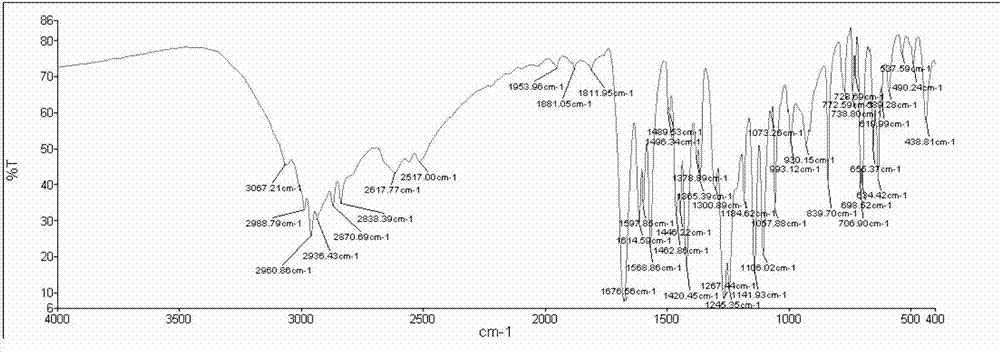
[0047] With a purity of 80% ( E )-2-Phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid crude product 10g (its HPLC diagram is shown in Figure 5 Shown) was added to 60mL of isopropanol and stirred to dissolve, then 0.1g of concentrated sulfuric acid was added dropwise, and the temperature was raised to 60°C. After reacting for 5 hours, cooled to room temperature, the alcohol sol...
Embodiment 2-6
[0056] Example 2-6 ( E )-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid purification method
[0057] Embodiment 2-6 is respectively a kind of ( E )-2-phenyl-3 (3,5-dimethoxy-4-isopropylbenzene) acrylic acid purification method is similar to the purification method of Example 1, the only difference is the technical parameters involved therein The specific differences are shown in the table below:
[0058]
[0059] Note: Substance A in the above table means ( E )-2-phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid; substance Aa means ( E )-2-phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid a; Substance A product represents the final ( E )-2-Phenyl-3(3,5-dimethoxy-4-isopropylphenyl)acrylic acid product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com