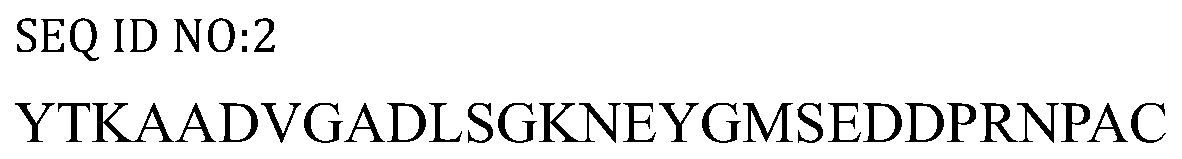A kind of monoclonal antibody hybridoma 3a6 of toxoplasma gondii protein tgvp1 and monoclonal antibody
A monoclonal antibody and hybridoma technology, applied in the field of cell engineering, can solve problems such as unclear mechanism of action, and achieve high specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
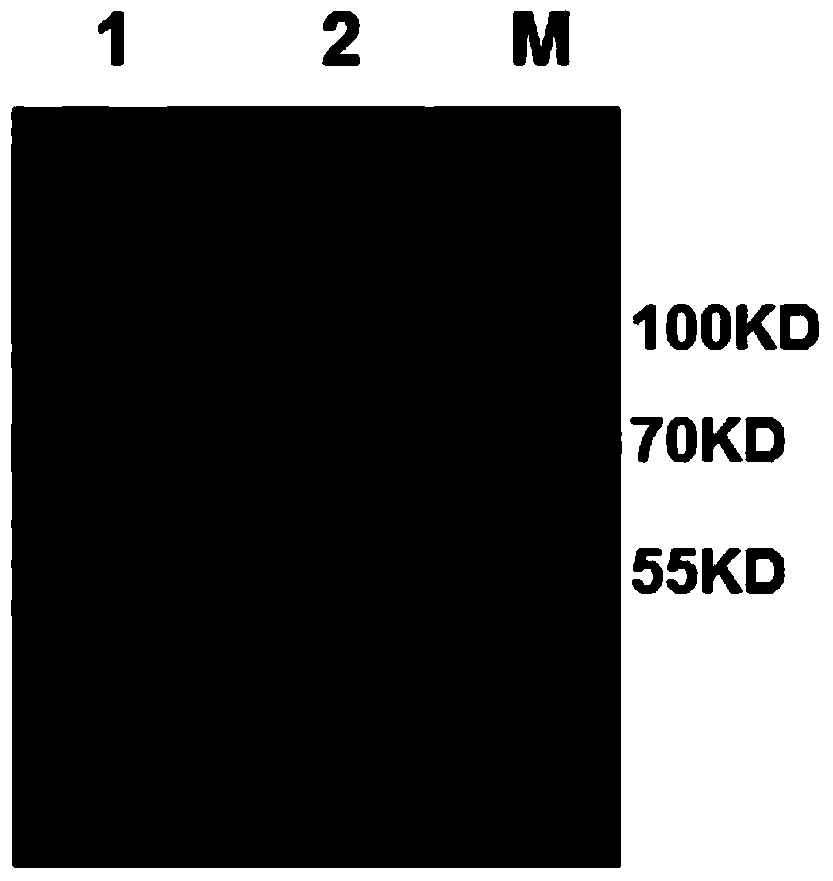
[0015] Example 1 Preparation and identification of the monoclonal antibody of the present invention
[0016] 1. Preparation of monoclonal antibody 3A6
[0017] 1) Design, preparation and carrier coupling of Toxoplasma gondii TgVP1 antigen polypeptide
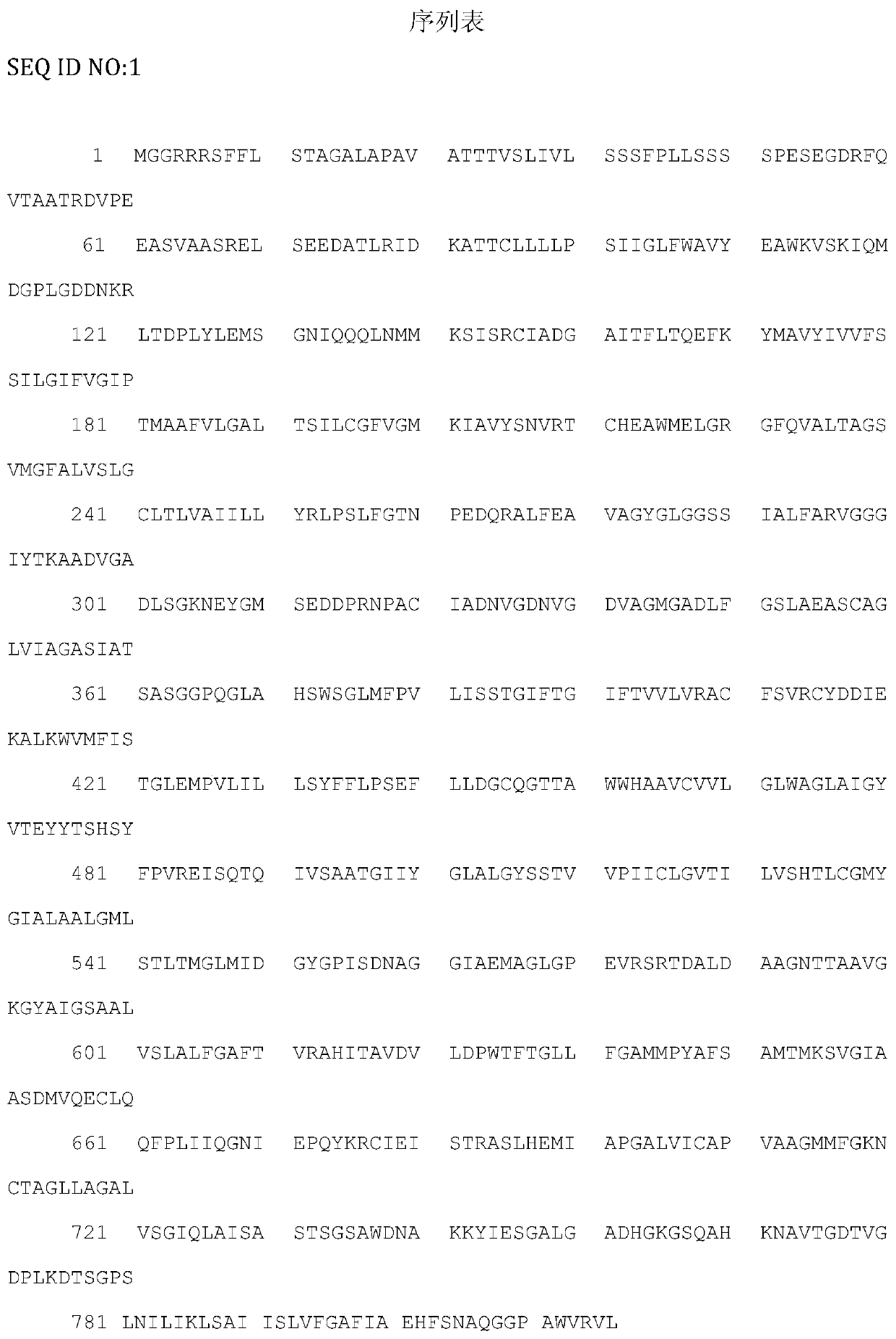
[0018] According to the Toxoplasma gondii TgVP1 sequence on GenBank (accession number: EPT25031.1), the Toxoplasma gondii TgVP1 protein sequence contains 816 amino acids. TMHMM software was used to analyze the transmembrane domain of TgVP1 protein, and IEDB software was used to analyze the immunogenicity, hydrophilicity and hydrophobicity and surface accessibility of TgVP1 protein. And strong. After the above analysis, the final screening polypeptide sequence is: 292aa-320aa, SEQ ID NO:2. The short peptide (TgVP1-P1) and the short peptide (TgVP1-P1-KLH) whose C-terminus is coupled to keyhole limpet hemocyanin carrier protein KLH were synthesized by Shanghai Gil Biochemical Company.
[0019] 2) Immune mice
[0020] 6-8 week ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com