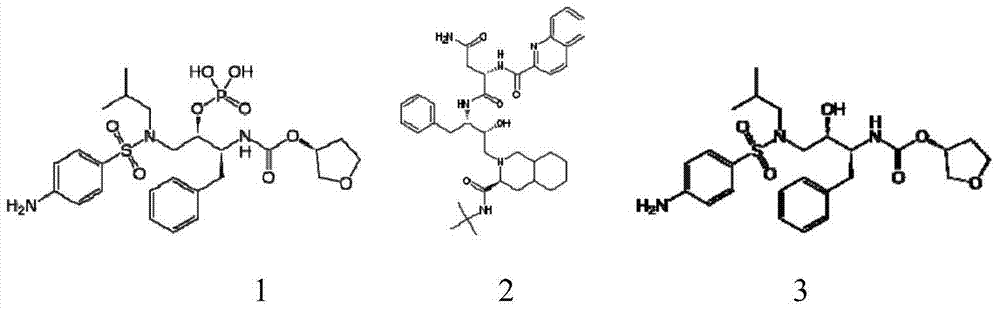
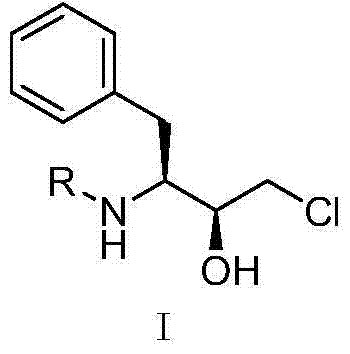
Preparation method of fosamprenavir intermediate
A system and hydrogen donor technology, applied in fermentation and other directions, can solve the problems of non-biological method reporting, and achieve the effects of short reaction time, low pollution and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The gene fragment (synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.) containing the ketoreductase gene (SEQ ID No.1-20) was ligated with the digested product of pET28a plasmid (purchased from Invitrogen), and transformed into competent E. The coli BL21 (DE3) strain was screened to obtain positive clones, which were inoculated into 4 mL of liquid LB medium containing ampicillin resistance and activated overnight (37° C., 200 rpm). From the overnight culture, transfer 100 mL of liquid LB medium containing ampicillin resistance at 1 / 100 inoculum, culture at 37°C and 200 rpm with shaking until the OD600 value reaches 0.6-0.8, add IPTG and continue culturing overnight at 30°C. The cells were collected by centrifugation, and suspended in 10 mL of phosphate buffer (2 mM, pH 7.0). The cell suspension was ultrasonically disrupted in an ice bath for 10 minutes, centrifuged, the supernatant was pre-frozen overnight, and freeze-dried for 24h-48h to obtain the recombinant ket...
Embodiment 2
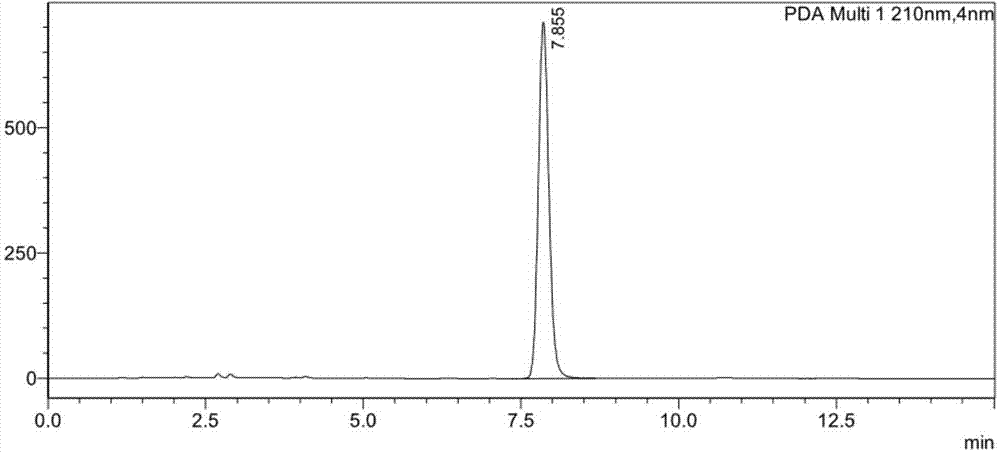
[0026] In a 10ml reactor, add 1.65mL 0.1M pH 7.0 phosphate buffer, dissolve 20mg of ketoreductase enzyme powder (SEQ ID No.1), 2mg of NADP or NAD in turn; substrate (N-protected (S)- 3-amino-1-chloro-4-phenyl-2-butanone) 0.1g dissolved in 0.25mL isopropanol, added to the reactor and stirred until completely dissolved, stirred at 1000rpm, reacted at 25°C for 24 hours, HPLC The conversion rate was detected, and the conversion rate of the NAD sample was 13.6%, and the conversion rate of the NADP sample was 61.3%.
Embodiment 3
[0028] In a 10ml reactor, add 1.65mL 0.1M pH 6.0-9.0 phosphate or triethanolamine buffer solution, dissolve 20mg of ketoreductase enzyme powder (SEQ ID No.1) and 2mg of NADP in sequence; the substrate (N-protected (S)-3-Amino-1-chloro-4-phenyl-2-butanone) 0.1g was dissolved in 0.25mL isopropanol, added to the reactor and stirred until completely dissolved, stirred at 1000rpm, and reacted at 25°C 20 hours, HPLC detects conversion rate, 6.0 (phosphate) sample conversion rate is 44.2%, 6.5 (phosphate) sample conversion rate is 52.8%, 7.0 (phosphate) sample conversion rate is 47.9%, 7.5 (phosphate) sample conversion rate The conversion rate was 56.3%, the conversion rate of the 8.0 (triethanolamine) sample was 57.3%, the conversion rate of the 8.5 (triethanolamine) sample was 62.1%, and the conversion rate of the 9.0 (triethanolamine) sample was 48.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com