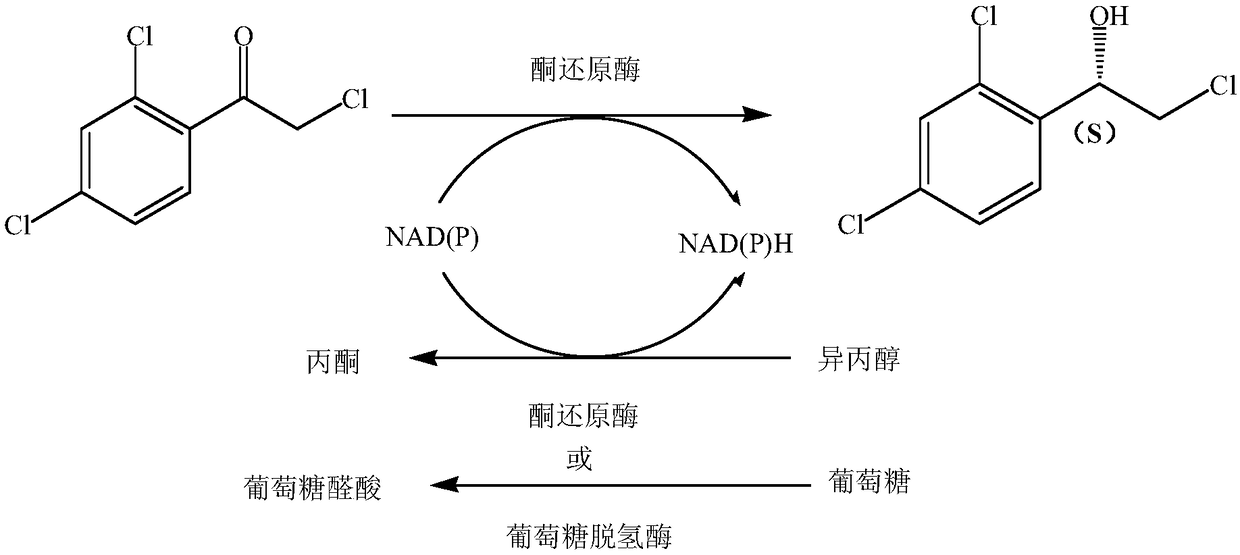
Enzymic preparation method of luliconazole intermediate
A technology for enzymatic preparation of azole intermediates, which is applied in the field of enzymatic preparation of luliconazole intermediates, can solve the problems of low chiral ee value and cannot meet industrial production, and achieves good optical purity, green environmental protection, low pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of the original gene of carbonyl reductase: according to the original gene sequence of carbonyl reductase LKADH, codon optimization was carried out on the original gene sequence according to the Escherichia coli codon analysis table, and the target gene was fully synthesized. The two ends were respectively cut with NdeI and XhoI sites and connected to pET26b(+) to obtain the recombinant expression vector pET26b-LKADH. The optimized LKADH original gene sequence is shown in SEQ NO.1, and its encoded amino acid sequence is shown in SEQ NO.2.
[0032] (2) Construction of carbonyl reductase mutants:
[0033] (3) Expression of carbonyl reductase: The recombinant expression vectors pET26b-LKADH and pET26b-LKADHM were transformed into expression strain BL21(DE3) by heat shock transformation method. Pick a single colony, insert the single colony into 5 mL of LB culture medium containing kanamycin, culture at 37°C overnight with shaking, take 1 mL of the bacterial ...
Embodiment 2
[0039]a) Synthesis of ketoreductase gene:
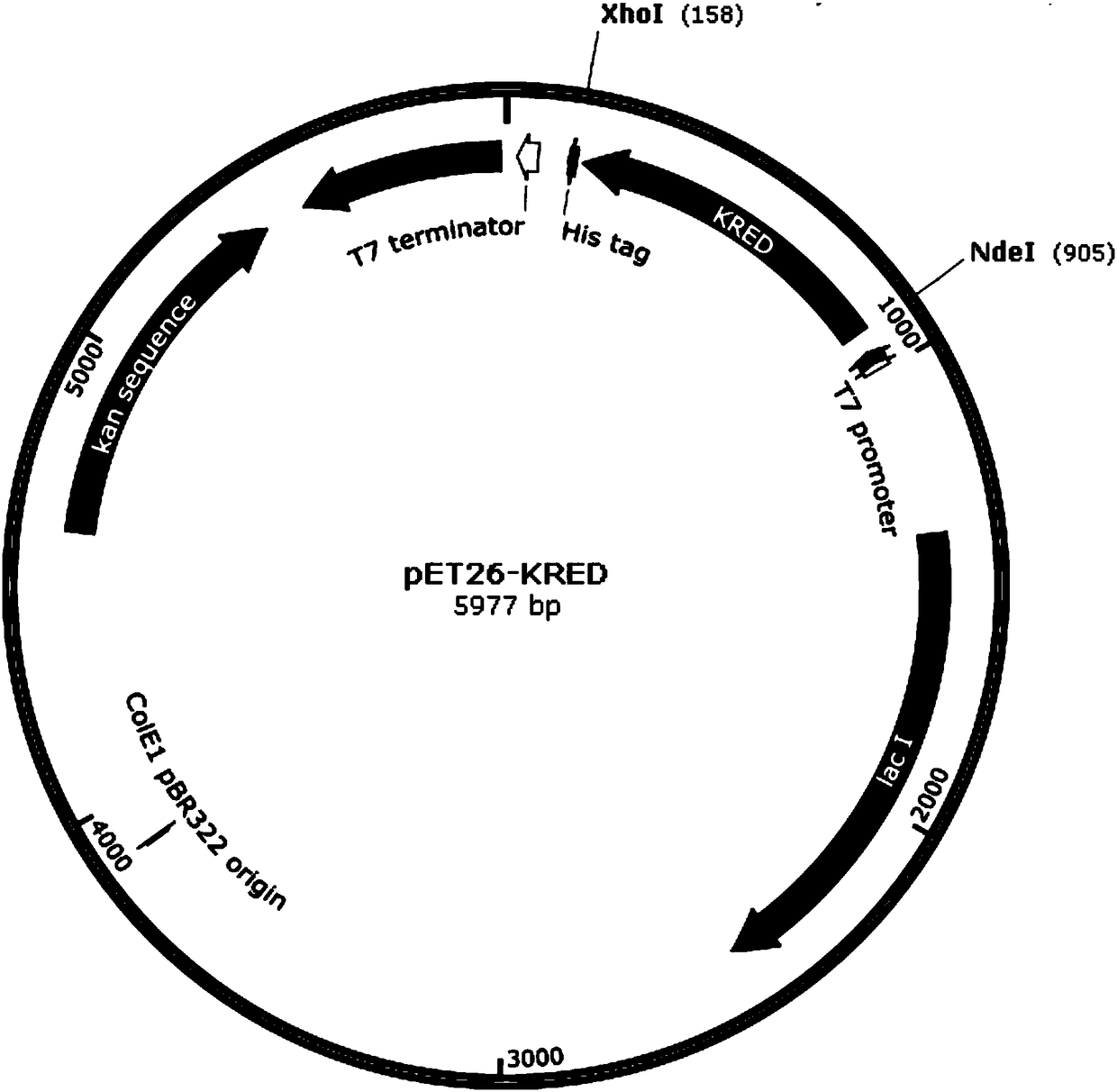
[0040] According to the codon preference of Escherichia coli, the ketoreductase gene KRED (SEQ ID NO.1) was synthesized from the whole gene, and Shanghai Jierui Biotechnology Co., Ltd. was entrusted to carry out the full synthesis of the gene. and XhoI restriction sites and ligated to pET26b(+) to obtain the recombinant expression vector pET26-KRED.
[0041] b) Transform the recombinant expression vector into Escherichia coli: Prepare Escherichia coli BL21 (DE3) competent cells, transform the recombinant expression vector pET26-KRED into BL21 (DE3) competent cells by calcium chloride method, and spread on LB plates containing 50 μg / mL of kanamycin resistance were incubated overnight at 37°C. Pick a single colony, extract the plasmid after a small amount of amplification, then perform 1.5% agarose gel electrophoresis, and observe it under ultraviolet light. The recombinant E. coli that contains the plasmid band is the recombinant E. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com