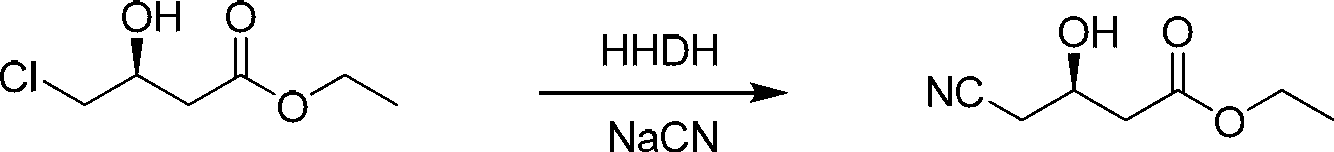
Biological preparation method of (R)-4-cyano-hydroxybutanoate
A technology of ethyl hydroxybutyrate and biological preparation, which is applied in the field of biopharmaceuticals and green chemistry, can solve the problems of high enzyme dosage, high enzyme feeding amount, and inappropriate reaction conditions, etc., and achieve reduced enzyme dosage, improved purity and yield , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Inoculate a single colony from a glycerol tube or transformation plate into 4 mL liquid LB medium containing ampicillin resistance and activate overnight (37°C, 200rpm). Transfer 100mL of liquid LB medium containing ampicillin resistance from the overnight culture at 1 / 100 (v / v) inoculum, culture with shaking at 37°C and 200rpm until the OD600 value reaches 0.6-0.8, add IPTG and continue to culture at 30°C overnight. Cells were collected by centrifugation, and suspended in 10 mL of phosphate buffer (2 mM, pH 7.0). The cell suspension was sonicated in an ice bath for 10 minutes. Centrifuge and pre-freeze the supernatant overnight. Freeze-dried for 24h-48h.
Embodiment 2
[0026] In a 50mL three-neck flask, add 23mL of deionized water and 7g of substrate in sequence; after stirring evenly, slowly drop into 5mL of 30% NaCN solution (20% sulfuric acid solution is used to maintain the pH of the system at 7.2 to 7.3 during the dropping process), and the halogenated alcohols are removed. Halogenase powder 35mg, at 50°C, pH 7.0 (30%NaCN solution titration), 800rpm magnetic stirring conditions, react for 24h, GC detection conversion rate> 98%, add sulfuric acid to adjust the pH to 2-3, add an equal volume of acetic acid Ethyl ester, diatomaceous earth filter, collect the organic phase, add an equal volume of ethyl acetate to the aqueous phase to extract twice, combine the organic phase, and rotary evaporate to obtain 6.3g of the product, with a purity of >98% and an optical purity of >99%.
Embodiment 3
[0028] Into the reactor, add 2.3L of deionized water and 700g of substrate in sequence; after stirring evenly, slowly drop into 0.5L of 30% NaCN solution (20% sulfuric acid solution is used to maintain the pH of the system at 7.2~7.3 during the dropping process), halogenated alcohol Dehalogenase powder 3.5g, at 50°C, pH 7.0 (30%NaCN solution titration), under the condition of mechanical stirring, react for 24h, GC detection conversion rate > 98%, add sulfuric acid to adjust the pH to 2-3, add an equal volume Ethyl acetate, filter with diatomaceous earth, collect the organic phase, add an equal volume of ethyl acetate to the water phase to extract twice, combine the organic phases, pass nitrogen gas through the lye to remove HCN gas, and rotary evaporate to obtain about 630g of the product, with a purity > 98%, optical purity >99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com