Preparation method of brookite titanium doxide nano-rod
A technology of titanium dioxide and brookite, applied in the direction of titanium dioxide, titanium oxide/hydroxide, nanotechnology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) 10ml of tetra-n-butyl titanate was added to 3.4ml of trifluoroacetic acid solution with a mass concentration of 50% for hydrolysis, wherein the molar ratio of tetra-n-butyl titanate: trifluoroacetic acid was 1:0.5, and it was heated violently at room temperature After stirring for 1 hour, the obtained tan solution was directly dried in the air at room temperature to remove volatile by-products to obtain a soluble xerogel;
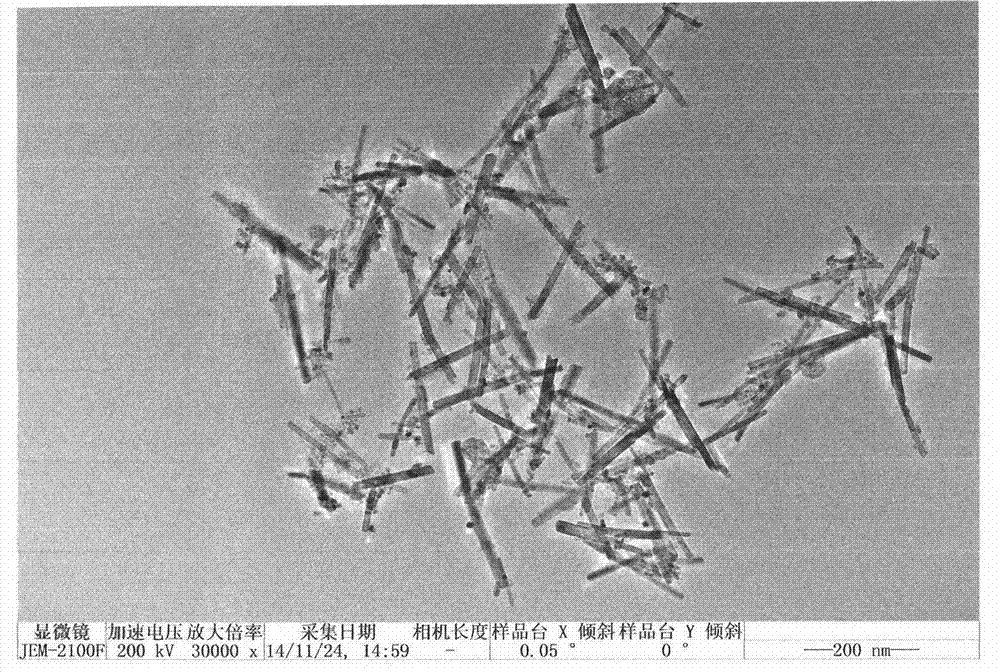
[0024] (2) Dissolve 0.5 g of the soluble xerogel obtained in step (1) in 20 mL of water, add 0.84 g of NaF to make the NaF concentration 1 mol / L, and then put the mixed solution into a polytetrafluoroethylene-lined stainless steel autoclave , 180 ℃ hydrothermal reaction for 24 hours. After the reaction, the precipitated product obtained is filtered, washed and dried to obtain brookite titanium dioxide nanorods. The prepared nanorods have a diameter of 14nm and a length of 220nm, such as figure 1 shown.
Embodiment 2
[0026] (1) 10ml of tetra-n-butyl titanate was added to 6.8ml of trifluoroacetic acid solution with a mass concentration of 50% for hydrolysis, wherein the molar ratio of tetra-n-butyl titanate: trifluoroacetic acid was 1: 1. After stirring for 1 hour, the obtained tan solution was directly dried in the air at room temperature to remove volatile by-products to obtain a soluble xerogel;
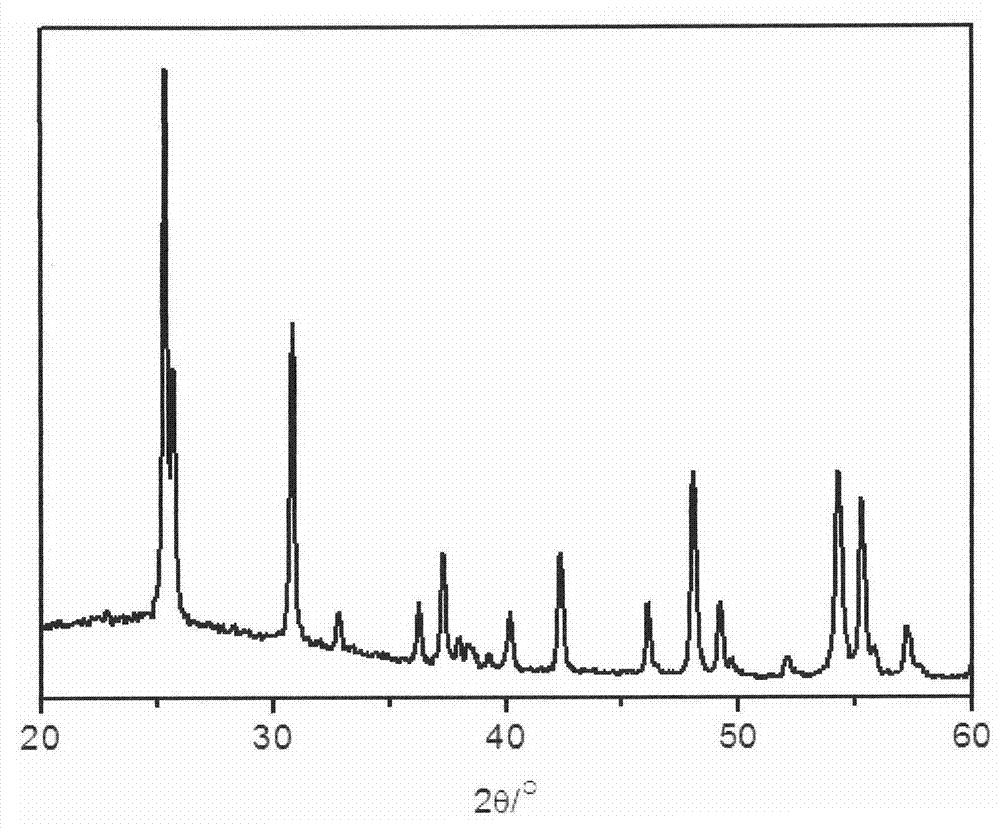
[0027] (2) Dissolve 0.5 g of the soluble xerogel obtained in step (1) in 20 mL of water, add 1.16 g of KF to make the KF concentration 1 mol / L, and then put the mixed solution into a polytetrafluoroethylene-lined stainless steel autoclave , 230 ℃ hydrothermal reaction for 48 hours. After the reaction, the precipitated product obtained is filtered, washed and dried to obtain brookite titanium dioxide nanorods. The prepared nanorods have a diameter of 20nm, a length of 300nm, and a purity of 98%, such as figure 2 shown.
Embodiment 3
[0029] (1) Add 10ml of tetra-n-butyl titanate to 13.6ml of trichloroacetic acid solution with a mass concentration of 75% for hydrolysis, wherein the molar ratio of tetra-n-butyl titanate: trichloroacetic acid is 1:3, and the After stirring for 1 hour, the obtained tan solution was directly dried in the air at room temperature to remove volatile by-products to obtain a soluble xerogel;
[0030] (2) Dissolve 0.8 g of soluble xerogel obtained in step (1) in 20 mL of water, add 2.12 g of Na 2 CO 3 , so that Na 2 CO 3 The concentration was 1 mol / L, and then the mixed solution was put into a polytetrafluoroethylene-lined stainless steel autoclave for a hydrothermal reaction at 180°C for 48 hours. After the reaction, the precipitated product obtained is filtered, washed and dried to obtain brookite titanium dioxide nanorods. The prepared nanorods have a diameter of 25nm, a length of 200nm, and a purity of 96%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com