Coenzyme Q10 intramuscular injection liquid and preparation method thereof
A technology of injection and coenzyme, applied in the field of biochemical medicine, can solve the problems of long time into the blood, inability to take oral administration, insecurity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
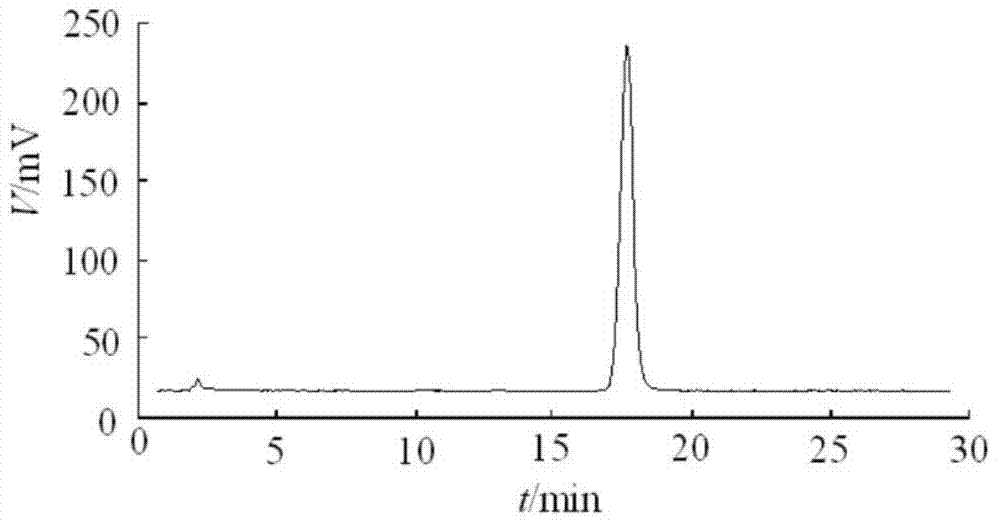
Embodiment 1
[0082] 1. Product formula:
[0083] Coenzyme Q10: 5g
[0084] Soybean oil: medium chain oil = 1:1 (mass ratio, the total amount is added to 2L)
[0085] Ve: 0.5% (mass percentage)
[0086] Benzyl alcohol: 2% (mass percentage)
[0087] 2. Preparation process:
[0088] 1) According to the formula described in claim 1, 2, 3, 4, 5, the vegetable oil is first heated to 60°C under the protection of high-purity nitrogen, then coenzyme Q10 is added, stirred until completely dissolved, and intermediate A is obtained;
[0089] 2) After cooling down at room temperature, check whether there is crystal precipitation. If there is precipitation, repeat step 1); if no precipitation, proceed to step 3);
[0090] 3) Add antioxidants, analgesics, and preservatives to Intermediate A in sequence, and stir until dissolved at room temperature to obtain Intermediate B;
[0091] 4) Intermediate B is passed through a 0.45 μm oily microporous membrane to remove invisible crystals and insoluble part...
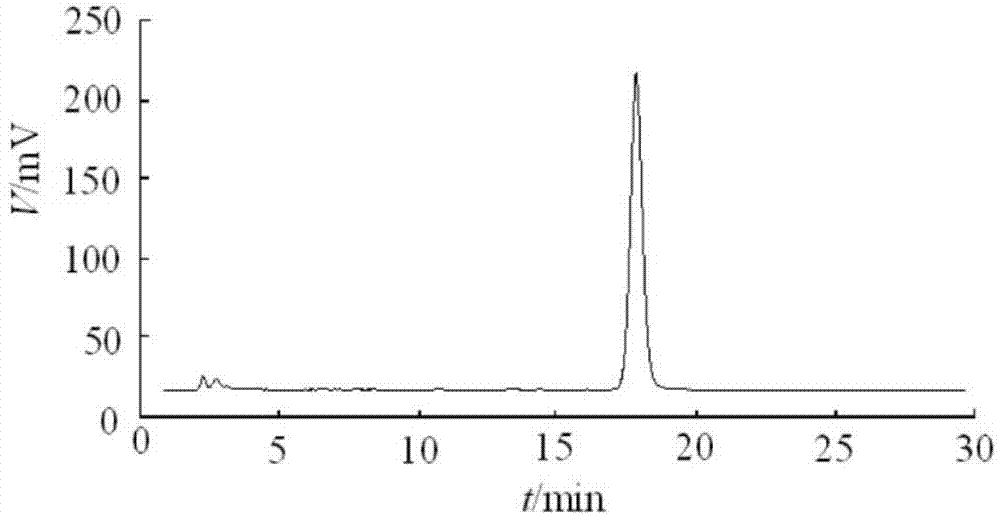
Embodiment 2
[0096] 1. Product formula:
[0097] Coenzyme Q10: 5g
[0098] Soybean oil: add up to 2L
[0099] Ve: 1% (mass percentage)
[0100] Benzyl alcohol: 2% (mass percentage)
[0101] 2. Preparation process
[0102] See Example 1.
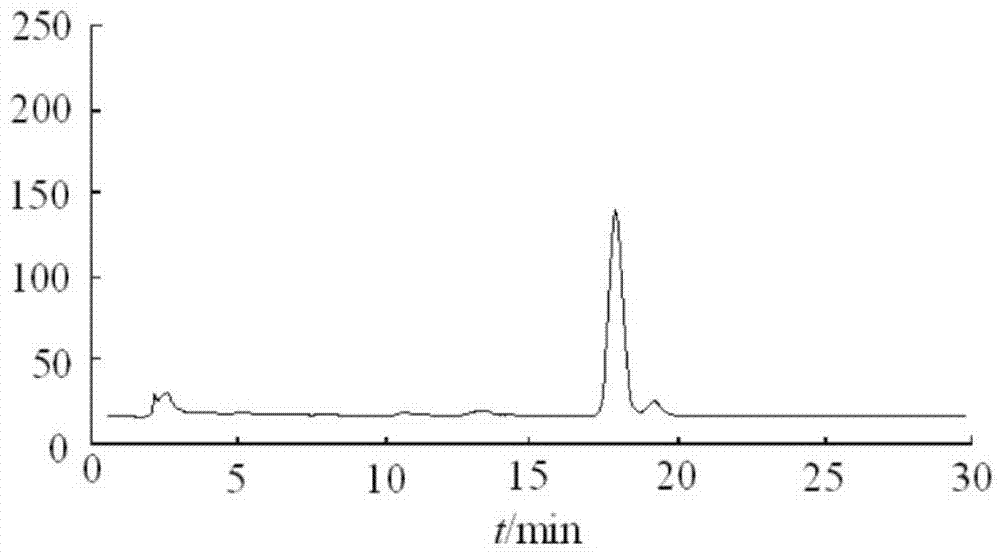
Embodiment 3
[0104] 1. Product formula:
[0105] Coenzyme Q10: 5g
[0106] Medium chain oil: add up to 2L
[0107] Ve: 1% (mass percentage)
[0108] Benzyl alcohol: 0.9% (mass percentage)
[0109] Benzoic acid: 0.5% (mass percentage)
[0110] 2. Preparation process
[0111] See Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com