Metal complexes of quinolinone derivatives, synthesis method and applications thereof
A technology of metal complexes and synthesis methods, which is applied in the field of medicine and can solve problems such as the synthesis and application of metal complexes that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: Synthesis of Co complexes with high-pressure solvothermal method
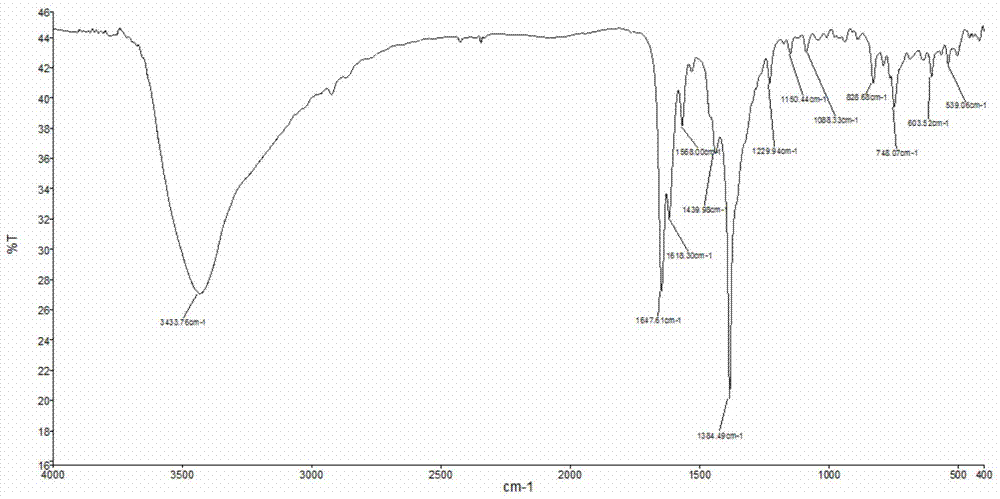
[0072] Add 0.1mmol Co(NO 3 ) 2 ·6H 2 O and 0.1mmol BMQ, then add 0.75ml methanol / chloroform mixed solution (the volume ratio of methanol and chloroform is 2:1). Under vacuum conditions, the open end was melted and sealed, and then fully reacted at 80° C. for 72 hours to obtain a red crystalline solid product. The product was subjected to infrared spectroscopy (e.g. Figure 4 shown), elemental analysis, electrospray mass spectrometry (such as Figure 5 shown) combined with X-ray single crystal diffraction analysis (such as Figure 6 Shown) for structure determination, identified as the target complex [Co(BMQ)(NO 3)(CH 3 OH)(H 2 O)] NO 3 . The structural formula is as follows:
[0073]
Embodiment 2
[0074] Embodiment 2: Synthesis of Rh complexes with high-pressure solvothermal method
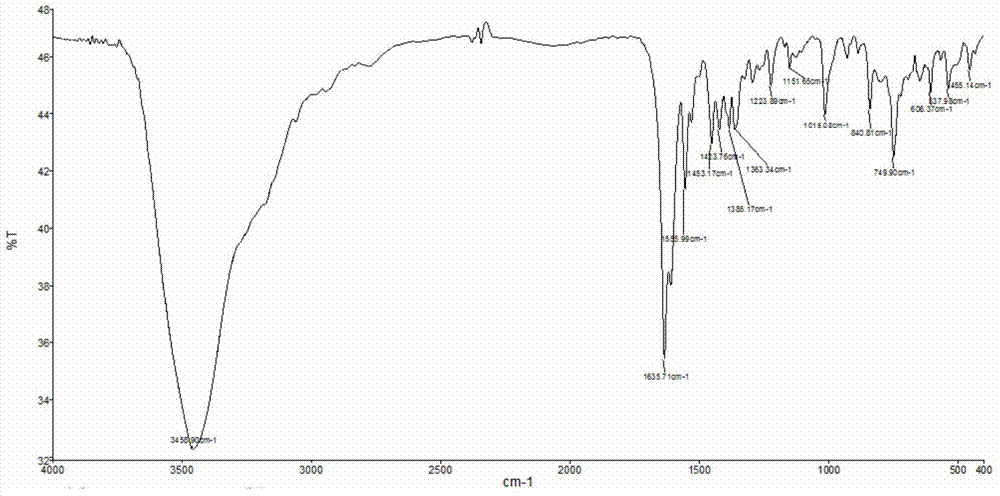
[0075] In a thick-walled borosilicate glass tube open at one end, directly add 0.1 mmol RhCl 3 ·3H 2 O and 0.1 mmol BMQ, and then add 0.6 ml methanol / DMF mixed solution (the volume ratio of methanol and DMF is 3:1). Under the condition of vacuuming, the open end was melted and sealed, and then fully reacted at 50° C. for 24 hours to obtain a red crystalline solid product. The product was subjected to infrared spectroscopy (e.g. Figure 7 shown), high-resolution mass spectrometry (such as Figure 8 shown), NMR spectrum (such as Figure 9 shown) and carbon spectrum (as Figure 10 shown), combined with X-ray single crystal diffraction analysis (such as Figure 11 shown) for structure determination, determined to be the target complex [Rh(BMQ)Cl 3 (CH 3 OH)]·CH 3 Oh. The structural formula is as follows:
[0076]
Embodiment 3
[0077] Embodiment 3: Synthesis of Ir complex with high pressure solvothermal method
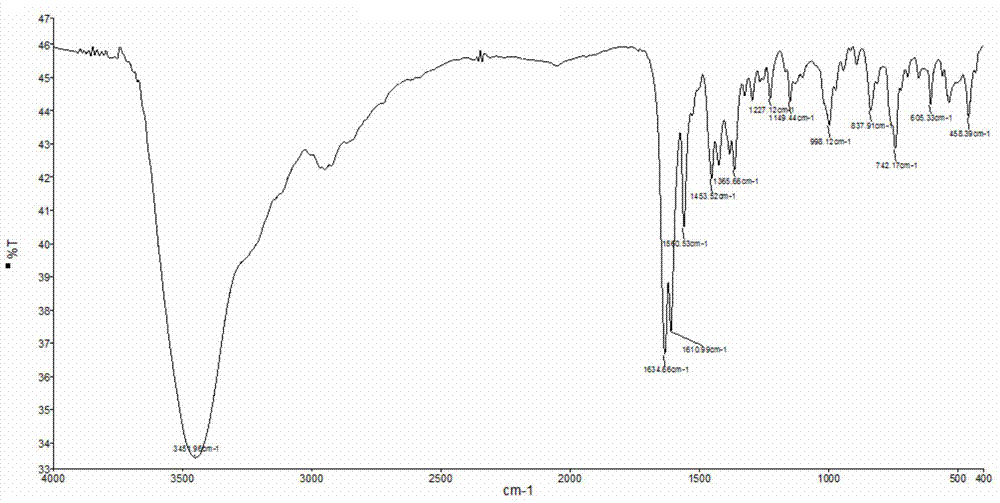
[0078] In a thick-walled borosilicate glass tube open at one end, directly add 0.1mmol IrCl 3 ·3H 2 O and 0.1mmol BMQ, and then add 0.6ml methanol-water mixed solution (the volume ratio of methanol and water is 3:1). Under the condition of vacuuming, the open end was melted and sealed, and then fully reacted at 100° C. for 50 hours to obtain a red crystalline solid product. The product was subjected to infrared spectroscopy (e.g. Figure 12 shown), high-resolution mass spectrometry (such as Figure 13 shown), NMR spectrum (such as Figure 14 shown) and carbon spectrum (as Figure 15 shown), combined with X-ray single crystal diffraction analysis (such as Figure 16 shown) for structure determination, determined to be the target complex [Ir(BMQ)Cl 3 (CH 3 OH)]·CH 3 Oh. The structural formula is as follows:
[0079]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com