Application of detecting pH of benzothiazole derivative in extremely acidic environment
A technology of benzothiazole and derivatives, applied in the field of benzothiazole compounds, can solve the problems of few pH fluorescent probes and limited types of pH fluorescent probes, and achieve the effects of low cost, good selectivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of probe (benzothiazole derivative) of the present invention:
[0028] (1) Under the protection of inert gas, 2-methylbenzothiazole (0.126ml, 1mmol), 4-(dimethylamino)cinnamaldehyde (0.263g, 1.5mmol) and KOH (0.354g, 6.25mmol) The reaction was stirred at room temperature in 7ml DMF for 24h.
[0029] (2) Remove the KOH solid by filtration, add H to the reaction solution 2 O (10ml), the mixed solution was extracted with dichloromethane. After the organic phase was dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure to obtain a crude product.
[0030] (3) The crude product was separated by silica gel column chromatography using dichloromethane as the eluent to obtain an orange-red solid. 1H NMR(300MHz,DMSO-d6):δ2.961-2.976(s,6H),6.674-6.893(m,3H),6.893-7.032(d,2H),7.290-7.476(m,5H),7.890- 8.022(d,1H),8.022-8.047(d,1H).MS: m / z 307.1264for M+.
Embodiment 2
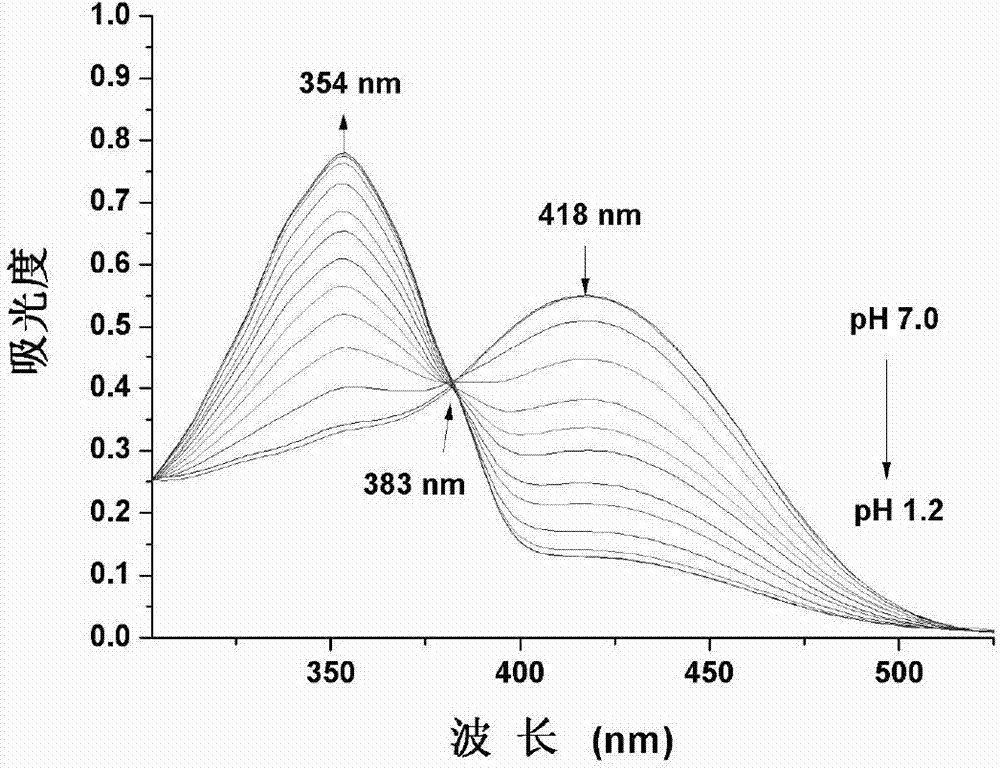
[0032] The probe in Example 1 was used to prepare a stock solution with a concentration of 1 mM in ethanol. In the experiment, the probe was diluted with water / ethanol (V / V=2 / 1) system to make the final concentration 10 μM. Regulate the pH value of this system with the HCl of 1mol / L, record the ultraviolet absorption spectrogram under different pH conditions ( figure 1 ). As the pH value decreased from 7.0 to 1.2, the absorption peak at 418nm decreased gradually, and the absorption peak at 354nm increased in turn, and there was an obvious isoelectric point at 383nm. figure 2 The color of the solution changed from yellow to colorless.
Embodiment 3
[0034]Similarly, the probe was diluted to 10 μM with water / ethanol (V / V=2 / 1) system for fluorescence spectrum titration, the excitation wavelength was fixed at 383 nm, and the fluorescence emission spectra under different pH conditions were recorded. Such as image 3 As shown, under the neutral condition of pH 7.0, the maximum fluorescence emission of the probe is located at 595nm. As the pH decreases, the fluorescence emission at 595nm gradually decreases, and a new fluorescence emission appears at 425nm and is significantly enhanced. At the same time, a clear isoelectric point appears at 522nm. Under the irradiation of ultraviolet light, the color of the solution changed from yellow to blue ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com