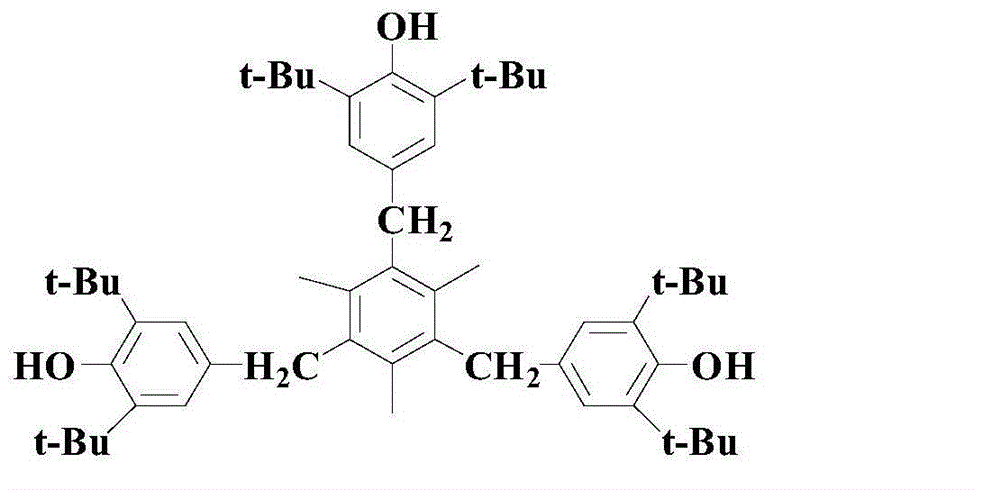
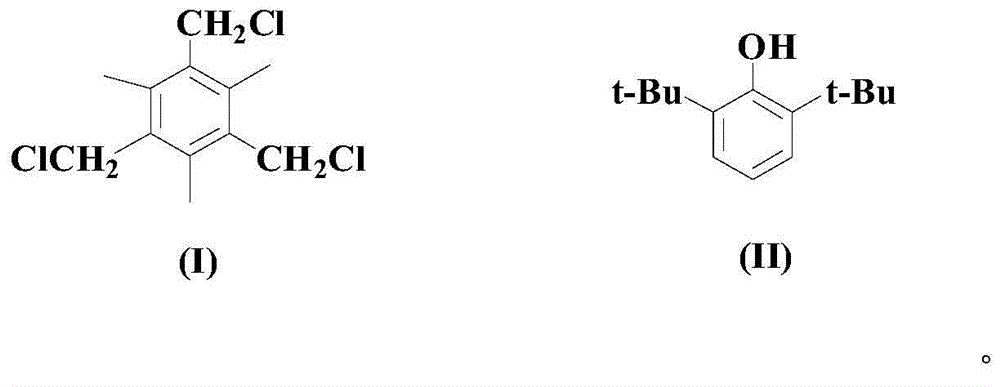Synthesis method for hindered phenolic compound antioxidant 330
A synthesis method and technology for hindered phenols, which are applied in the field of organic chemical synthesis, can solve the problems that the catalyst types need to be developed, the reaction process needs to be simplified, the reaction yield needs to be improved, etc., and the effect of good application potential and prospects can be achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0044] Preparation Example 1: Preparation of Carbon Nanotube-supported Acid Catalyst
[0045] S1: adding carbon nanotubes to a sufficient amount of concentrated nitric acid with a concentration of 90% by mass, heating to reflux for 35 minutes, filtering, fully washing with deionized water, and drying in a vacuum oven to obtain acidified carbon nanotubes;
[0046] S2: adding the acidified carbon nanotubes obtained in step S1 into absolute ethanol, and fully stirring until a suspension is formed; wherein the mass volume ratio of the acidified carbon nanotubes to absolute ethanol is 1:4g / ml ;
[0047] S3: Add tetraisopropyl titanate Ti(OC) dropwise to the suspension obtained in step S2 3 h 7 ) 4 , continue to stir while adding dropwise until it becomes a sol, then fully dry and pulverize to obtain powder; wherein the mass of the acidified carbon nanotubes (ie, the acidified carbon nanotubes obtained in step S1) and tetraisopropyl titanate The ratio is 1:0.2;
[0048] S4: Add...
preparation example 2
[0049] Preparation Example 2: Preparation of Carbon Nanotube-supported Acid Catalyst
[0050] Except that the acidification treatment of step S1 was not carried out, preparation example 2 was carried out in the same manner as preparation example 1, that is, the carbon nanotubes were not subjected to acidification treatment with concentrated nitric acid, but the treatment of step S2 was directly carried out, and the obtained carbon nanotubes were The supported acid catalyst was named T2.
preparation example 3-8
[0051] Preparation Example 3-8: Preparation of Carbon Nanotube-supported Acid Catalyst
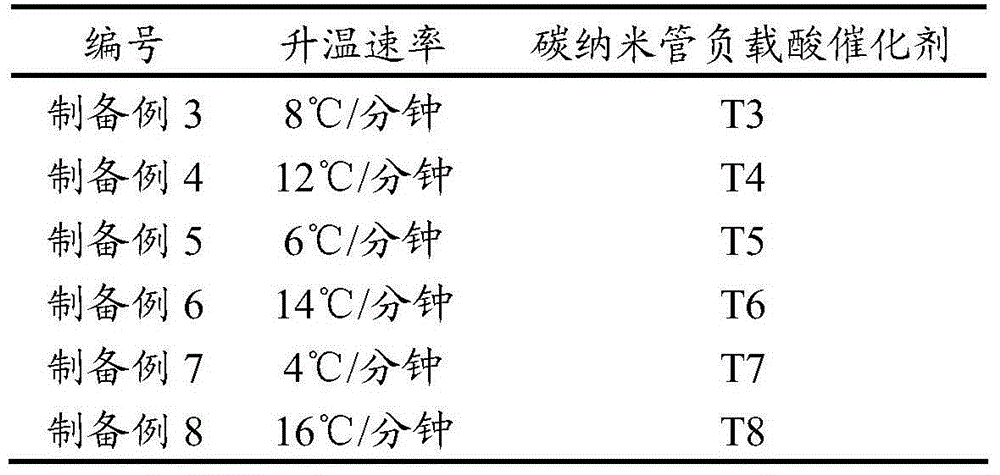
[0052] Except changing the temperature rise rate in step S4, other all remain unchanged, carry out preparation example 3-8 with the same embodiment as preparation example 1, the gained carbon nanotube supported acid catalyst is named as T3-T8 in sequence; Wherein temperature rise rate, resulting catalyst see Table 1 below Shown:
[0053] Table 1 .Carbon nanotube-supported acid catalysts obtained at different heating rates
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com