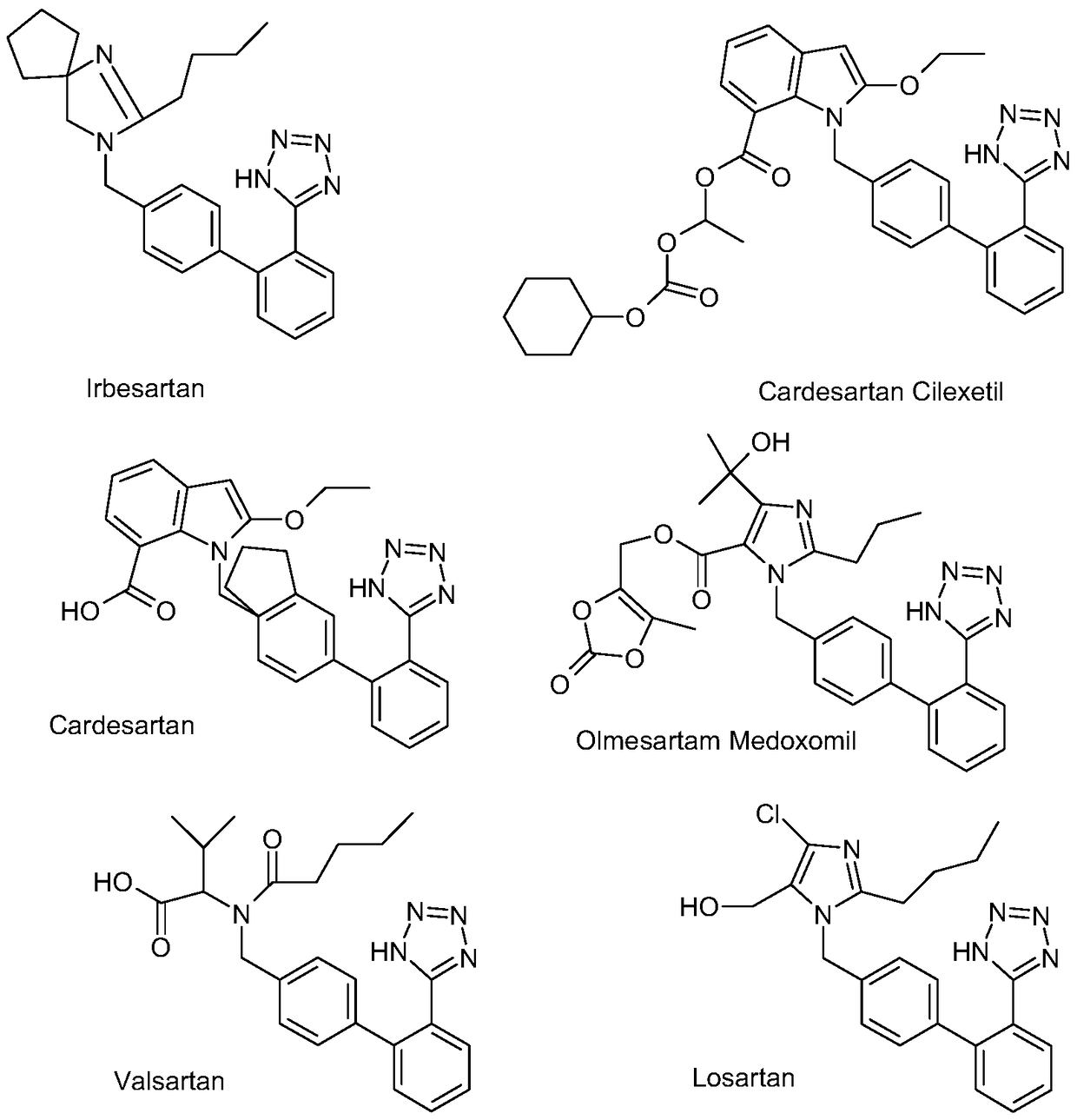
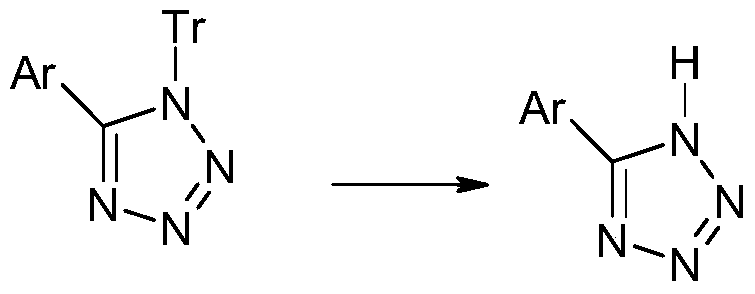
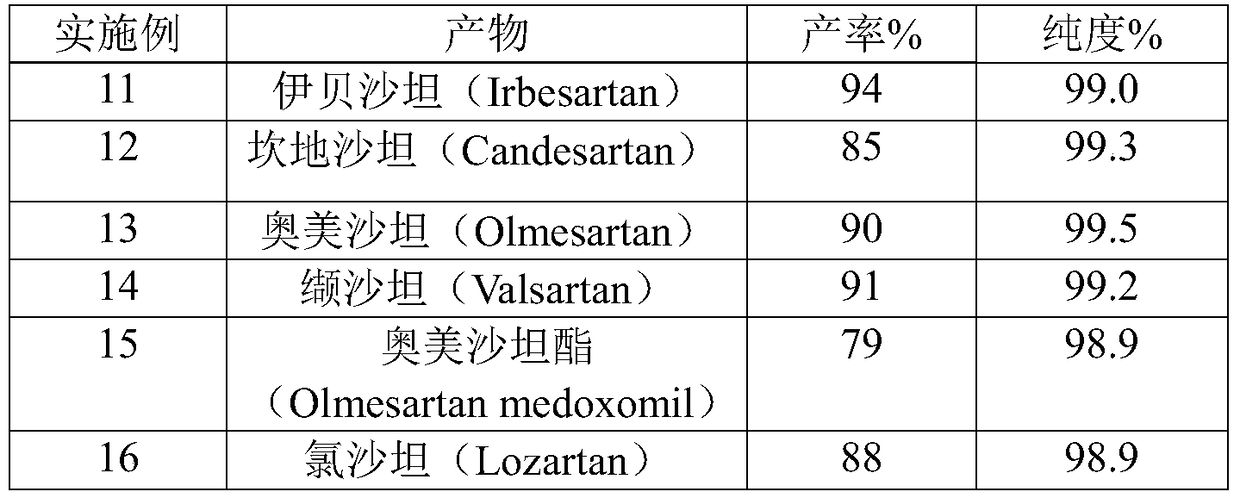
A method for preparing sartan drugs by removing trityl protecting group
A technology of trityl and sartan, which is applied in the field of drug synthesis, can solve the problems of long reaction time, impurity, unsatisfactory yield, and low product purity, and achieve the effects of easy recycling, easy purification, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Candesartan Cilexetil
[0033] (1) Add 409.2mL of dichloromethane, trityl candesartan cilexetil (42.6g, 0.050mol), 136.4mL of anhydrous methanol and 13.6g of montmorillonite to a 1L reaction flask, and the temperature is raised to 38~42℃ Reflux for 4-24 hours, until the HPLC content of trityl candesartan cilexetil is detected by HPLC <2.0% is the end of the reaction. The reaction solution was filtered, and the filter cake was washed with 68.2 mL of dichloromethane. Combine the filtrate, cool to 20-25°C, add 409.2 mL of water, adjust pH=4.5-5.5 with 1% dilute hydrochloric acid, and stir for 10 min. After standing for 30 min, the separated organic layer was washed twice with 409.2 mL of water.
[0034] (2) The organic layer was evaporated to dryness under reduced pressure. Add methyl tert-butyl ether (MTBE, 341.0 mL) preheated to 35~40℃, add a little seed crystal, cool to 15~25℃, stir for 10h, and filter. The obtained wet product was slurried with ...
Embodiment 2~5
[0037] The operation of Examples 2 to 5 is basically the same as that of Example 1, except that a different solvent is used instead of methylene chloride for the reaction during the reaction; or a different solvent is used instead of methyl tert-butyl ether for beating during post-treatment. The reaction conditions and results are shown in Table 1.
[0038] Table 1 Reaction conditions and test results of Examples 2 to 5
[0039] Example
[0040] a Only use methanol as solvent, reaction time is 25h
Embodiment 6~10
[0042] The operations of Examples 6 to 10 are basically the same as that of Example 1, except that other insoluble acids are used as catalysts instead of montmorillonite for the reaction. The reaction conditions and results are shown in Table 2.
[0043] Table 2 Reaction conditions and test results of Examples 6-10
[0044] Example
catalyst
Yield%
purity%
6
94
99.3
7
Pretreated diatomaceous earth a
94
99.3
8
H-ZSM-1
95
99.2
9
93
99.2
10
no b
86
98.9
[0045] a Before using the diatomaceous earth, stir it with 5% hydrochloric acid for two hours, filter, wash with water until it is neutral, and then dry;
[0046] a The reaction time is 28h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com