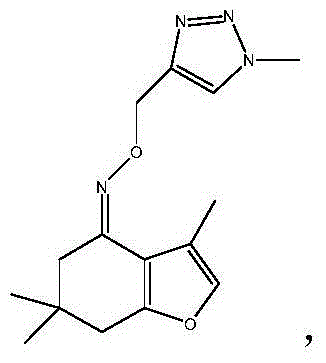
Tetrahydrobenzofuran-4-ketoximetriazole medicine, and preparation method and application thereof
A technology of ketoximo-based triazole and benzofuran is applied in the field of synthesis of tetrahydrobenzofuran-4-ketoximo-based triazole anti-gastric cancer drugs, and can solve the problems of lack of treatment means and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
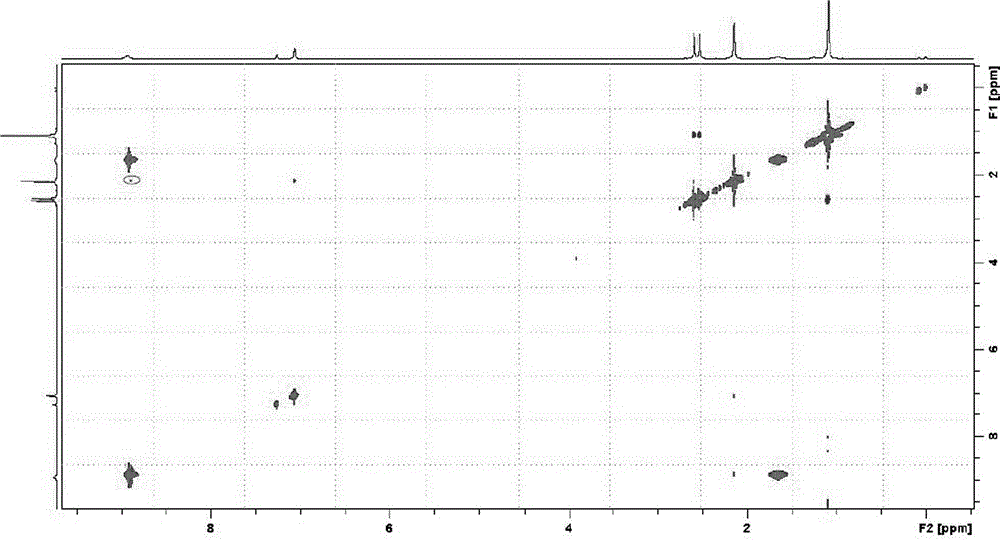
[0080] First, 3,6,6-trimethyl-6,7-dihydrobenzofuran-4(5H)-one (intermediate 1) and azide (intermediate 2) were prepared, and then the intermediate 1 is converted into cis-ketoxime (intermediate 3), after separation and purification, it is alkylated to obtain propargyl oxime ether (intermediate 4), and finally intermediate 4 and azide intermediate 2 are synthesized by copper-catalyzed ring Addition reaction to synthesize 1,2,3-triazolyl-substituted furanocyclohexanone oxime derivatives, the synthetic route is as follows:
[0081]
[0082] Synthesis of azide (intermediate 2): p-Hydroxyacetophenone (1.36g, 10.0mmol) was dissolved in ethyl acetate (20ml), then copper bromide (4.48g, 20.0mmol) was added to the solution, heated Reflux for 5 hours. After cooling to room temperature, filter, pour 100mL of water into the filtrate, extract with ethyl acetate (3×100mL), separate the layers, dry the organic phase with anhydrous sodium sulfate, filter with suction, and remove the solve...
Embodiment 2
[0088] Other steps are the same as in Example 1, in the synthesis steps of target compounds 5a-5h, react propargyl ether (intermediate 4, 0.5 mmol) with 2-azidoarylethanone in a tert-butanol-water mixed system, The reaction control and separation and purification methods are the same as in Example 1, only a different type of azide intermediate 2 needs to be replaced. Azide compounds are 2-azide aryl ethyl ketone, azide arene, aryl methylene azide
Embodiment 3
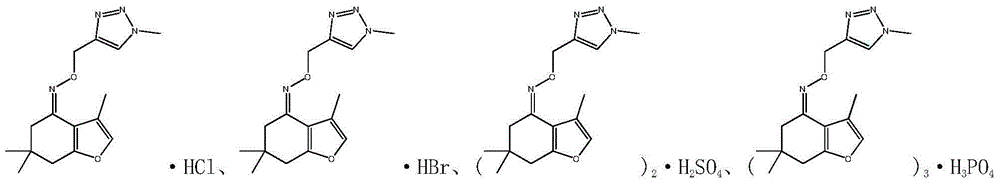
[0090] The preparation method of tetrahydrobenzofuran-4-ketoxime triazolium salt takes hydrochloride as an example. Weigh 1.0 mmol of one of the target products 5a to 5h in a 50 mL three-necked bottle, dissolve it in 5 mL of anhydrous methanol, cool the reaction bottle to 0 ° C under the protection of nitrogen, and add 1 mol / L of hydrogen chloride / methanol solution, stirred until the product was completely salified (thin-layer chromatography monitors the salination process of neutral molecules), evaporated to dryness, and obtained tetrahydrobenzofuran-4-ketoximotriazole hydrochloride.
[0091] The preparation method of hydrobromide, sulfate, oxalate etc. is the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com